By Tiziana Vavalà, MD, and
Silvia Novello, MD, PhD
Posted: April 2018
Approximately 1.8 million new lung cancer cases worldwide are diagnosed annually, and 85% of these cases are NSCLC. Only 25% to 30% of patients with NSCLC are suitable for potentially curative resection. Despite optimal surgical management and postoperative treatments, nearly one-third of patients with stage I NSCLC and at least 30% to 50% of those with stages II and III disease will still die from recurrent disease.1,2
Lung adenocarcinoma is the most commonly diagnosed histologic subtype of NSCLC.3 Introduction of targeted therapies, specifically those directed toward EGFR active mutations and ALK rearrangement, have improved outcomes in a subset of patients with advanced NSCLC harboring those alterations; however, the role of molecular testing and targeted therapies for earlier-stage NSCLC remains unclear, and practice varies worldwide.4,5
The ALCHEMIST Platform
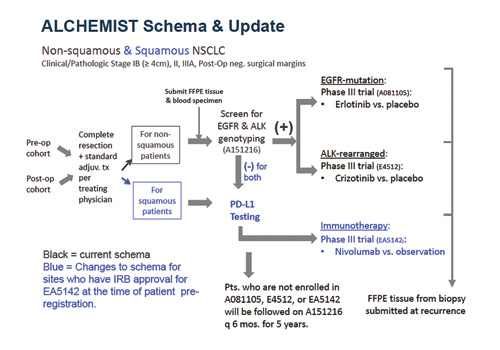
The Adjuvant Lung Cancer Enrichment Marker Identification and Sequencing Trial (ALCHEMIST) applies the umbrella design to identify and screen patients with EGFR active mutations and ALK rearrangement in early-stage resected non-squamous NSCLC through three integrated protocols6, with further trials anticipated or planned in the future. Patients with squamous NSCLC have been subsequently planned to be included for those sites who had Institutional Review Board/Ethics Committee approval for ALCHEMIST-Immunotherapy (EA5142) trial at the time of patient pre-registration (Figure 1).
In the ALCHEMIST SCREENING (A151216) trial, patients with nonsquamous (and squamous for approved sites) stage Ib (> 4 cm)-IIIA NSCLC are evaluated and considered for one of the ALCHEMIST therapeutic protocols. As part of this trial, non-squamous tumor samples and blood specimens are genotyped for EGFR mutations and ALK rearrangement and are collected for whole-exome or -genome analysis. If either of these molecular drivers are present, patients are subsequently referred to one of two treatment trials (ALCHEMIST-EGFR [A081105] or ALCHEMIST-ALK [E4512]), which are testing erlotinib (for EGFR mutations) or crizotinib (for ALK rearrangement) versus observation in patients with completely resected non-squamous NSCLC after standard therapy. Patients with squamous histology and those who are negative for EGFR or ALK alterations undergo PD-L1 testing and are eventually referred to the Nivolumab After Surgery and Chemotherapy in Treating Patients With Stage IB-IIIA Non-small Cell Lung Cancer (ANVIL) trial (ALCHEMISTImmunotherapy [EA5142]). All patients who are not enrolled in previous subtrials will be monitored on A151216 for relapse and survival for 5 years.
Improved Clinical Trial Efficiency
The usual drug-development paradigm of phase I followed by phase II and phase III to define a clinical benefit is challenged by the presence of target therapies and the incorporation of biomarker assessment for medical treatment.7 Enrichment, umbrella, and basket trial designs are gaining popularity; they offer novel strategies to accelerate the drug-development process, with the goal of delivering personalized therapies to eligible patients more quickly. Among innovative trial designs, the umbrella infrastructure usually has the flexibility to add (or drop/modify) subtrials of molecularly targeted drugs and companion diagnostics based on accumulating evidence from the ongoing trial or newly emerging data elsewhere.8 The ALCHEMIST platform, with its current design, significantly enhances the prospect of cure, particularly if positive results emerge from these biomarker-driven trials. ALCHEMIST has prompted a new vision of lung cancer though a genomic landscape, and it is defining a systematic approach of cure in a molecularly defined subset of patients.8
This approach to clinical research is even more relevant in Europe, where delays in access to anticancer drugs and to molecular testing have postponed the choice of a target approach for patients with oncogene addiction. For example, crizotinib, the ALK first-generation TKI for first-line treatment of patients with ALK-rearranged NSCLC, was approved for reimbursement in Italy only recently (in February 2017) whereas the U.S. Food and Drug Administration (FDA) granted an accelerated approval in August 2011.9,10 This delay has limited therapeutic options for eligible patients over the past 4 years and has also compromised the proper implementation of detection testing in clinical practice.
This consideration is supported by recent data from the Italian Observational Prospective Trial. In this trial, 1,787 patients with advanced or recurrent NSCLC were evaluated between November 2014 and November 2015. Of the participants, 1,351 (75.6%) presented with adenocarcinoma, 280 (15.7%) presented with squamous carcinoma, and the rest presented with mixed or poorly differentiated histologies; 1,353 patients (76%) were tested for EGFR, but only 942 (53%) were evaluated for ALK. As stated by the authors, this was probably due to the costs of analysis (fluorescence in situ hybridization was often the main, or only, tool applied for ALK translocation); without a fully reimbursed first-line ALK inhibitor, clinicians opted to postpone the analysis, giving priority to the EGFR test in a daily clinical practice.11 Moreover, in the same study, it was clearly shown that only 707 (40%) patients received a second-line treatment. It is well known that after first-line therapy, the general conditions of patients with lung cancer may dramatically worsen, inducing clinicians to start a classical second-line treatment rather than asking and waiting for a molecular report or repeating a tissue biopsy.11 As a consequence, a high percentage of patients lose the opportunity to receive appropriate second-line targeted therapy. ALCHEMIST is, in any case, a proactive attempt to avoid the same mistakes in early-stage NSCLC.
All cited issues highlight the profound and challenging effects of ALCHEMIST, not only in Europe, but also from a global perspective. ALCHEMIST is a strong academic effort to improve clinical trial efficiency and the first opportunity to systematically characterize the presence of molecular changes in the early-stage setting, using innovative technologies to translate research data early in the drug-development process and improve adjuvant treatment. The ALCHEMIST approach could be a valuable way to answer the need for a better, science-based, consensual position on methodology, inspiring greater commitments from politicians and healthcare decision makers to ensure broad, quick, and equal access to innovative antitumor drugs. ✦
About the Authors: Dr. Vavalà is Oncologist and Referent for Thoracic Malignacies at SC of Oncology at ASL CN1, Saluzzo Hospital, Italy. Prof. Novello is Professor of Medical Oncology at Department of Oncology at San Luigi Hospital, University of Turin, Italy.
References:
1. Le Chevalier T. Adjuvant chemotherapy for resectable non-small-cell lung cancer: where is it going? Ann Oncol. 2010;21(Suppl 7):vii196– vii198.
2. Vansteenkiste J, Crinò L, Dooms C, et al. 2nd ESMO Consensus Conference on Lung Cancer: early-stage non-small-cell lung cancer consensus on diagnosis, treatment and follow-up. Ann Oncol. 2014;25(8):1462-1474.
3. Travis WD. Pathology of lung cancer. Clin Chest Med. 2011;32:669-692.
4. Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362:2380-2388.
5. Kwak EL, Bang YJ, Camidge DR, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med. 2010;363(18):1693-1703.
6. Govindan R, Mandrekar SJ, Gerber DE, et al. ALCHEMIST Trials: A Golden Opportunity to Transform Outcomes in Early Stage Non–Small Cell Lung Cancer. Clin Cancer Res. 2015;21(24): 5439-5444.
7. Mandrekar SJ and Sargent DJ. Drug designs fulfılling the requirements of clinical trials aiming at personalizing medicine. Chin Clin Oncol. 2014;3(2):14.
8. Mandrekar SJ, Dahlberg SE, Simon R. Improving Clinical Trial Efficiency: Thinking outside the Box. Am Soc Clin Oncol Educ Book. 2015:e141-e147.
9. Italian Pharmacy Agency website. http://www.gazzettaufficiale.it/atto/serie_generale/ caricaDettaglioAtto/originario;jsessionid=TPUWixBXpN-cg-T2ravbA__.ntc-as1-guri2a?atto.dataPubblicazioneGazzetta=2017-02-24&atto.codiceRedazionale=17A01289&elenco30giorni=false Accessed January 17, 2018.
10. Crizotinib. National Cancer Institute website. cancer.gov/about-cancer/treatment/drugs/crizotinib. Accessed January 6, 2018.
11. Gobbini E, Galetta G, Tiseo M, et al. Molecular profiling in Italian patients with advanced non-small-cell lung cancer: An observational prospective study. Lung Cancer. 2017 Sep;111:30-37.