By Beth Eaby-Sandy, MSN, CRNP
Posted: February 2018
When I started in thoracic medical oncology 15 years ago, we just had chemotherapy, and patient education was focused and streamlined. Chemotherapy could be toxic and life threatening, but we had specific warnings and guidelines to give to patients. We now have chemotherapy, targeted therapies, and immunotherapy drugs. Patient education has changed from a one-size fits-all approach to one that must be tailored based on the class of drug.
Immunotherapy in NSCLC can present a challenge for patient education because oft en the patient has already endured chemotherapy and now expects the same side effects and risks to continue. Often, there is a sense of relief when a patient hears how well tolerated immunotherapy usually is, and that there is a much lower risk of nausea, hair loss, and lowered blood counts. In most instances, this is the case; however, 5% to 10% of patients who receive immunotherapy will experience significant immune-mediated adverse events.
These include, but are not limited to:
• Pneumonitis (seen more frequently in patients with NSCLC as opposed to other tumor types)
• Colitis
• Endocrinopathies (thyroid, adrenal, and pituitary)
• Rash/dermatitis
• Hepatitis/nephritis
There are several other less common immune-mediated toxicities that can occur, including several neurologic, hematologic, and cardiac toxicities. As an oncology community, we are still discovering more immune-mediated toxicities as these drugs become more widely used.
Management is based around the use of systemic steroids along with other supportive-care measures specific to each adverse event. There are several different management protocols recommended by clinicians, as well as the pharmaceutical industry. The National Comprehensive Cancer Network, American Society of Clinical Oncology, Society for Immunotherapy of Cancer, and others are working together to create a consensus- based clinical management strategy. The sidebar contains details of the most commonly seen toxicities and some management strategies that can be used. Th ere are several other less-common toxicities that can manifest with many different patient symptoms. In general, I tell patients that if they are feeling unwell or if something doesn’t feel right, they should call and report the symptoms and let the oncology team decipher symptoms and related approaches.
The IASLC Nursing and Allied Health Committee has produced an immunotherapy toxicity identification and management tool, which is a great clinical piece to refer to while seeing patients with these toxicities. (For more information, see iaslc.org/toxicities or email [email protected].) As an oncology community, we will continue to learn about these toxicities and how to better identify and manage them. Proper patient education can lead to early detection of immune-mediated adverse events, mitigation of which is important before the events become life threatening. ✦
About the Author: Ms. Eaby-Sandy is a Nurse Practitioner at Abramson Cancer Center, University of Pennsylvania.
Common Toxicities (All Grades) and Their Associated Management Strategies
Pneumonitis
• Frequency with PD-1/PD-L1 inhibitors: 2% to 4%.
• Patients should immediately report: Increase in shortness of breath or nagging frequent cough.
• What it looks like on imaging: See Fig. 1A and Fig. 1B.
• Treatment: Steroids per recommended guidelines – generally oral prednisone at high doses (0.5-1.0 mg/ kg)1 with a minimum of 4-week taper schedule or intravenous (IV) corticosteroids if inpatient; consult with pulmonology; oxygen if required; and immune suppression when indicated for severe cases.
Fig. 1A. Patient Who Is Non-Symptomatic, Grade 1
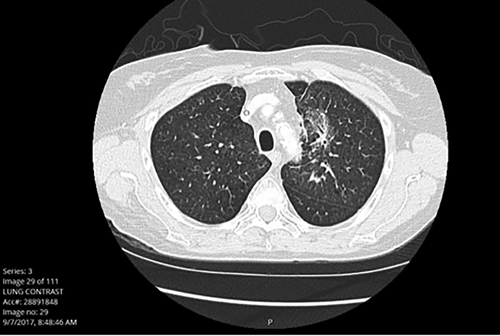
Fig. 1B. Patient Who Is Significantly Symptomatic, Unable to wean off of Steroids, Grade 3
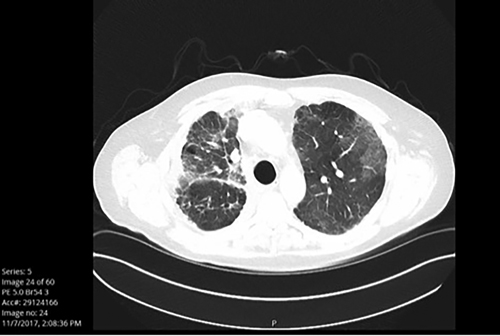
Colitis
• Frequency with PD-1/PD-L1 inhibitors: 1% to 3% (19% diarrhea/colitis reported for atezolizumab).
• Patients should immediately report: Worsening diarrhea associated with abdominal pain, cramping, or blood and/or mucus in the stool.
• What it looks like: Consider colonoscopy for diagnosis, otherwise, based on patient symptoms.
• Treatment: Steroids – generally oral prednisone at high doses (0.5-1.0 mg/kg)1 with a minimum of 4-week taper schedule or IV corticosteroids if inpatient; consult with gastrointestinal (GI) specialist if not improving; antidiarrheal agents; stool culture; and immune suppression when indicated for severe cases. Endocrinopathies
• Frequency with PD-1/PD-L1 inhibitors: Up to 10%.
• Patients should immediately report: Heart palpitations, severe malaise, intolerance to temperature, loss of appetite, or increased thirst/urination. Oft en there are no real symptoms.
• What it looks like: Th yroid-stimulating hormone signifi cantly up or down; adrenocorticotropic hormone levels or cortisol levels signifi cantly depleted; brain MRI for suspected hypophysitis; fasting glucose if diabetes is considered.
• Treatment: Th yroid replacement; hormone replacement for depleted cortisol levels; monitoring labs; insulin for diabetes, and immune suppression when indicated for severe cases.
Dermatitis
• Frequency with PD-1/PD-L1 inhibitors: 1% to 9%.
• Patients should immediately report: Significant rash with hives or significant itching with or without rash.
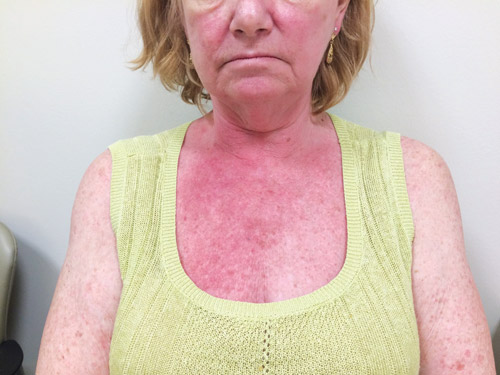
• What is looks like: Typically erythematous, papular, on face, neck, and/or chest. Rarely pustular. Fig. 2 shows a rash from a PD-1 inhibitor. Rashes from anti–CTLA-4 drugs alone or combined with PD-1 inhibitors typically can cause more severe dermatitis.
• Treatment: Topical steroid creams; antipruritics; sunscreen; and oral steroids in severe cases only.
Fig. 2. Rash from a PD-1 Inhibitor
Nephritis and Hepatitis
• Frequency with PD-1/PD-L1 inhibitors: 1% to 5%
• Patients should immediately report: Decrease in urine output. Most patients will be asymptomatic unless sudden, severe inflammation occurs.
• What is looks like: Lab values show elevations in liver function tests or renal function.
• Treatment: Steroids (sometimes pulse for nephritis), generally oral prednisone at high doses (0.5–1.0 mg/kg)1 with a minimum of 4-week taper schedule or IV corticosteroids if inpatient; further immune suppression in severe cases; consult GI or renal services for input. ✦
Note: % of toxicities are taken directly from the product inserts for pembrolizumab, nivolumab, and atezolizumab. Numbers reported are from all studies across different tumor types, but may be higher or lower depending on specific trials.
Read the article: Immunotherapy Adverse-Event Treatment Resource Guide
Reference:
1. Boutros C, Tarhini A, Routier E, et al. Safety profiles of anti-CTLA-4 and anti-PD-1 antibodies alone and in combination. Nat Rev Cancer. 2016;13(8):473-486.











