By John K. Field, PhD, and Matthijs Oudkerk, MD, PhD
Lung cancer kills more Europeans than any other cancer. In 2014, 272,000 citizens of the twenty-eight countries in the European Union (EU) died from lung cancer, which is 20.1% of all cancer deaths.1 When implemented in the general population, lung cancer screening using CT saves lives.
The European lung cancer CT screening community involved in randomized controlled trials has a long, collaborative history. In the past 12 to 15 months, this group of experts across multiple clinical disciplines in lung cancer CT screening— involving eight countries—decided that it needed to provide leadership and direction to the European policy makers on how lung cancer screening should be implemented.
The expert group is fully aware that lung cancer screening has already started in the United States (using the U.S. Preventive Services Task Force’s recommendation2) and that funding has been agreed to by Medicare. However, Europe is sitting on the fence while opportunistic lung cancer screening has already started.
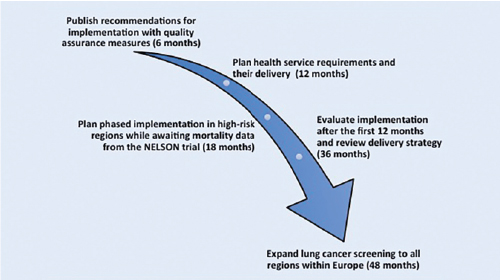
The EU position statement on lung cancer screening (EUPS), published in December 2017 in The Lancet Oncology,3 has provided a set of nine recommendations on how to take lung cancer CT screening forward in Europe, dealing with many of the outstanding questions that were posed after the 2011 National Lung Screening Trial publication (see Recommendations).4,5 The EUPS expert opinion now argues that lung cancer specialists in Europe must start planning for implementation, as outlined in Fig. 1.3
Clearly, there are a number of major issues that must be resolved prior to implementation. These include, but are not limited to, (1) how the high risk population is identified for screening—either general practitioner records or national questionnaires—and the use of risk prediction models; (2) service provision of CT scanners for screening; (3) training of radiologists and radiographers; and (4) whether the radiology community will set up central reading resources and, if so, how quality assurance will be performed.
The expert group estimates that the above organizational issues will take approximately 18 months to resolve, and the group also recognizes that it is highly likely that the Dutch-Belgian Lung Cancer Screening trial (NELSON) will publish results during this 18-month timeframe.5 NELSON results will be key because it is the largest European randomized lung cancer screening trial and was designed to investigate whether low-dose CT screening can reduce lung cancer mortality by 25% or greater when compared with no screening after 10 years of follow-up.6
Fig. 1. EU Position Statement on Lung Cancer CT Screening ‘Call for Action’
It should also be noted that the recommended protocol for the management of pulmonary nodules has been updated based mainly on the recent NELSON publications, as outlined in Recommendation 6: “Management of prevalent lung nodules in CT screening programmes, lung nodules at incident screening (newly detected), and CT-detected lung nodules in clinical practice should be managed with different protocols because of different pretest lung cancer probabilities.”3
The EU lung cancer screening community considers the EUPS policy review to be an important planning document on how to best implement lung cancer screening in Europe, and the policy review will be discussed with the health policy makers throughout the EU. Policy makers in Europe are on the verge of making a definitive decision regarding whether to implement lung cancer screening, but, unfortunately, none of the countries in Europe are currently prepared to move forward practically. ✦
About the Authors: Professor John K. Field is the Director of the Roy Castle Lung Cancer Research Programme, Department of Molecular and Clinical Cancer Medicine at The University of Liverpool in the United Kingdom. Professor Matthijs Oudkerk is with University Medical Center Groningen at the University of Groningen in The Netherlands.
| Recommendations
1. Low-dose CT is the only evidence based method for the early detection of lung cancer shown to provide a mortality reduction. On the basis of this evidence from randomized controlled trials, the EU position statement recommends that we start to plan for the implementation of lung cancer screening in Europe while cognizant of future publications that include the awaited NELSON trial data on mortality and cost-effectiveness and data from the six smaller European studies for developing implementation strategies in each of their own countries. 2. Future lung cancer low-dose CT programs should use a validated risk stratification approach so that only individuals deemed to be at high enough risk are screened. In the near future, incorporation of potential biomarkers and susceptibility genes into lung cancer risk models should be considered to improve the accuracy of risk stratification models. 3. All future screenees entering into early detection programs for lung cancer should be provided with carefully constructed participant information on the potential benefits and harms of screening to enable them to make an informed decision as to whether they wish to participate or not. Smoking cessation advice should be offered to all active smokers. 4. Future management of screen-detected solid nodules should utilize semi-automatically derived volume measurements and volume-doubling time, and should be quality assured. 5. National quality assurance boards should be set up by professional bodies to ensure adherence to all minimum technical standards, including semi-automated volumetry, and to standardize diagnostic criteria for screen-detected lung nodules, including radiation exposure limits. 6. Management of prevalent lung nodules in CT screening programs, lung nodules at incident screening (newly-detected), and CT-detected lung nodules in clinical practice should be managed with different protocols because of different pretest lung cancer probabilities. 7. Although only evidence for annual low-dose CT lung cancer screening is available, recent research suggests the possibility of using a more personalized approach to lung cancer screening with a risk-based approach on the results of baseline and first screening rounds. 8. Management of lung nodules by lung cancer multidisciplinary teams should be done according to the EU position statement recommendations with the aim of minimizing harm and ensuring patients receive the most appropriate treatment. 9. The EU position statement expert group recommends that the planning for low-dose CT screening should be started throughout Europe because low-dose CT lung cancer screening has the potential to save lives. ✦ Reprinted with permission of The Lancet. |
Read the article: An American Perspective on the European Lung Cancer Screening Implementation Plans
References:
1. Eurostat. Cancer statistics—specific cancers. http://ec.europa.eu/eurostat/statistics-explained/index.php/Cancer_statistics_-_specific_cancers#Lung_ cancer. Accessed December 19, 2017.
2. U.S. Preventative Services Task Force. https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/lung-cancer-screening. Accessed December 14, 2017.
3. Oudkerk M, Devaraj A, Vliegenthart R, et al. EU Position Statement on Lung cancer Screening. Lancet Oncol. 2017;18(12):e754-e766.
4. National Lung Screening Trial Research Team, Aberle DR, Adams AM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365(5):395-409.
5. Field JK, van Klaveren R, Pedersen JH, et al. European randomized lung cancer screening trials: Post NLST. J Surg Oncol. 2013;108(5):280- 286.
6. van Iersel CA, de Koning HJ, Draisma G, et al. Risk-based selection from the general population in a screening trial: selection criteria, recruitment and power for the Dutch-Belgian randomised lung cancer multi-slice CT screening trial (NELSON). Int J Cancer 2007:120(4):868-874.











