Posted: December 2017
Experts in molecular testing discussed various issues on this topic in October at the IASLC 18th WCLC. Neal Lindeman, MD, led off the session with an overview of the update to the College of American Pathologists (CAP), International Association for the Study of Lung Cancer (IASLC), and the Association for Molecular Pathology (AMP) 2013 molecular testing guideline in lung cancer.
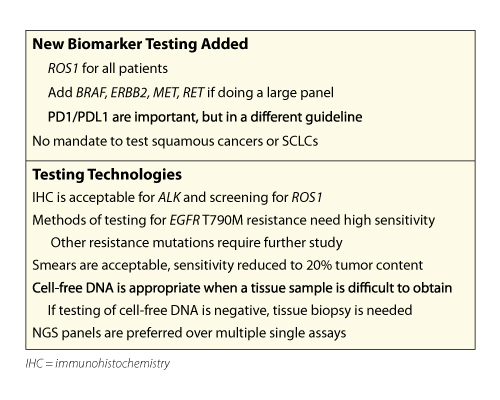
Dr. Lindeman explained that updating guidelines at least every 5 years is standard practice. He discussed several factors that influenced the current update, including the emergence of new biomarkers and targeted therapies; advances in testing technology, such as next-generation sequencing (NGS) and blood-based liquid biopsy; and reconsideration of squamous cancers and small cell lung cancers (SCLCs) Dr. Lindeman reviewed the draft recommendations (Table 1), noting that the 2013 recommendations remain largely unchanged. He explained that ROS1 testing is recommended and should be performed on all patients with advancedstage adenocarcinoma, irrespective of clinical characteristics. Molecular testing for BRAF, RET ERBB2 (HER2), MET, and KRAS is not currently indicated as a routine stand-alone test outside the context of a clinical trial; however, it is appropriate to include these as part of larger testing panels performed either initially or when routine EGFR, ALK, and ROS1 testing is negative.
Revisions of the guideline are based on evidence from an unbiased review of published experimental literature since 2013 and include recommendations from an expert panel of renowned, worldwide leaders in the field. A draft of the guidelines was available for open comment during the development process, and the update is on schedule to be published in the Journal of Thoracic Oncology, the Journal of Molecular Diagnosis, and Archives of Pathology & Laboratory Medicine. ✦