By Cynthia L. Kryder, MS
Posted: December 2017

The IASLC WCLC 2017 Presidential Symposium in Yokohama, Japan, recognized the authors of 3 exceptional lung cancer research abstracts. Patient-reported Outcomes from the PACIFIC Trial According to previously reported efficacy and safety results of the PACIFIC trial, patients with locally advanced stage III NSCLC receiving durvalumab post completion of definitive concurrent chemoradiation had a significant improvement in progression-free survival (16.8 months vs. 5.6 months) (Figure 1), and a lower incidence of new lesions, including new brain metastases, compared with patients receiving placebo. Additionally, durvalumab was well tolerated with a manageable safety profile. Rina Hui, MBBS, FRACP, PhD, Crown Princess Mary Cancer Centre, Westmead, Australia, presented additional data on patient-reported outcomes, a secondary endpoint of the PACIFIC trial, a randomized, placebo-controlled, double-blind, phase III study in locally advanced, unresectable NSCLC.
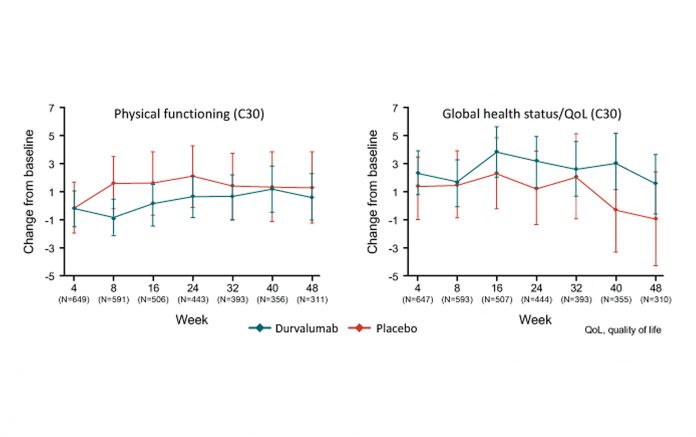
Dr. Hui and colleagues randomly assigned 713 patients who had previously received 2 or more cycles of platinum-based concurrent chemotherapy with definitive dose radiation without disease progression to durvalumab (476 patients) or placebo (237 patients) for up to 12 months. Key symptoms, physical function, and global health status/quality of life were assessed using the EORTC QLQ-C30 v3 questionnaire and its lung cancer module, QLQLC13. Changes from baseline were analyzed using a mixed model for repeated measures. Dr. Hui reported that compliance for questionnaire completion was above 80% in both groups and there were no between-group differences at baseline for key symptoms, physical function, or global health status/quality of life. She stated that treatment with durvalumab after concurrent chemoradiation therapy did not worsen symptoms, function, or global health status/quality of life for patients with locally advanced, unresectable NSCLC. Similarly, she noted that the change from baseline for key symptoms was minimal with both durvalumab and placebo (change in score from baseline <±10).
“Combined with the efficacy and safety findings from PACIFIC, these data further support the use of durvalumab in this disease setting,” concluded Dr. Hui.

Discussant Michael Boyer, MBBS, PhD, Sydney Cancer Centre, Sydney, Australia, commented that measuring patient-reported outcomes is a vital component of clinical trials such as PACIFIC to ensure that a survival improvement is not outweighed by a negative impact on quality of life. Mature Survival Results in a Spanish Lung Cancer Group Trial Bartomeu Massuti, MD, Alicante University Hospital, Alicante, Spain, presented the mature survival results of SCAT, a randomized phase III multicenter trial that examined whether it might be possible to customize adjuvant chemotherapy based on BRCA1 expression in patients with resected node-positive NSCLC.
Dr. Massuti explained that BRCA1 and BRCA2 are important DNA repair factors primarily involved in the repair of double-strand DNA breaks. BRCA1 efficiency has been found to enhance resistance to cisplatin, whereas loss of BRCA1 function is associated with sensitivity to DNA-damaging chemotherapy. SCAT investigators hypothesized that a significant proportion of patients with high BRCA1 expression would be cisplatin-resistant and would benefit from single-agent docetaxel.
A total of 500 patients were randomly assigned to the control (108 patients) or experimental arm (392 patients). Patients in the control arm received docetaxel plus cisplatin. In the experimental arm, 110 patients with low BRCA1 expression levels received cisplatin and gemcitabine, 127 patients with intermediate levels received cisplatin and docetaxel, and 110 patients with high levels received docetaxel alone. Overall survival was the primary endpoint. Dr. Massuti reported that customization of adjuvant chemotherapy according to BRCA1 levels did not yield significant differences in overall survival for the overall population in node-positive resected NSCLC. In the subgroup with low BRCA1 levels, experimental treatment with cisplatin/gemcitabine proved superior to the control group (74 months vs. 40.1 months). In addition, survival for the subgroup with high BRCA1 expression levels receiving treatment without platinum was similar to that for the control group. Dr. Massuti noted that higher BRCA1 expression levels were associated with male sex, squamous histology, and current or former smoking status.
Discussant Joan Schiller, University of Texas-Southwestern Medical Center, Dallas, US, remarked that relatively few DNA repair-driven clinical trials have examined the role of BRCA1 in lung cancer, as Dr. Massuti and colleagues have done. She noted that, although SCAT was negative for its primary endpoint of survival in the experimental arm compared with the control arm, it does provide evidence that patients with high BRCA1 expression levels have a worse prognosis and that BRCA1 status may serve well as a stratification factor in future trials. In addition, BRCA1 expression might be even more important in determining the role of taxanes rather than platinating agents.
IASLC Lung Cancer Staging Project: Analysis of Resection Margin Status and Proposals for R Status Descriptors
The residual tumor (R) classification, which describes tumor status after surgery, reflects the effectiveness of treatment, has prognostic impact, and may affect further treatment. John Edwards, MD, University of Sheffield, Sheffield, United Kingdom, reported on the efforts of the IASLC R Domain Subcommittee to analyze existing and potential R status criteria, including the 2005 proposed IASLC definition for “uncertain” resection margin status using data collected for the IASLC Lung Cancer Staging Project. Dr. Edwards reported the results based on 14,712 patients undergoing surgery for NCSLC for whom full R status and survival data were available.
“In designing and analyzing clinical trials
of adjuvant therapies, undertaking a
thorough evaluation and characterization
of R status is critical” – John Edward, MD
Dr. Edwards and colleagues reviewed data and reassigned cases to the uncertain category [R(un)] if any of the following parameters were present: examination of fewer than 3 N1 or N2 nodes; less than lobe-specific systematic lymph node dissection; extracapsular invasion of N2 nodes; positive highest lymph node station; carcinoma in situ at bronchial resection margin; and positive pleural lavage cytology. Revised categories of R0, R(un), R1, and R2 were designated and tested for survival impact.
Dr. Edwards reported that 56% of cases became R(un) after recoding. The majority of cases were R(un) due to intraoperative staging that proved less rigorous than systematic lymph node dissection. He noted that in pN2 cases with the highest station positive, median survival was 14 months less compared with highest station-negative cases. In node-positive cases, median survival was 20 months less for those with R(un) status compared with R0.
Dr. Edwards stated that these results show that the IASLC Proposed Definition for Complete Resection has relevance, according to the 8th Edition Database. High-quality surgical staging, he indicated, gives the most accurate assignment of stage group and the most favorable survival data, stage by stage. Likewise, optimal staging data enable the most appropriate decision-making for routine adjuvant therapy.
“In designing and analyzing clinical trials of adjuvant therapies, undertaking a thorough evaluation and characterization of R status is critical,” concluded Dr. Edwards. “Further detailed data collection will be necessary to see the full impact of R status subcategories in a clinical setting, and we urge institutions to participate in the IASLC Lung Cancer Staging Project. Our confirmation of the IASLC’s proposed criteria is an important step in promoting high-quality intraoperative staging and detailed pathologic assessment.” Discussant Kemp Kemstine, MD, PhD, University of Texas- Southwestern Medical Center, Dallas, US, noted that these data confirm that R(un) is problematic, as half of the cases had an uncertain complete resection and in this group survival was poor, although better than R1 and R2. He remarked that in the database of 94,708 patients, only 15.5% of patients had sufficient information for analysis. In the future, it would be important to determine what components or combinations of components in the IASLC database are problematic. ✦
Editor’s Note: This article is modified and reprinted from IASLC WCLC 2017
Daily News.