By Cynthia L. Kryder, MS, CCC-Sp
Posted: October 2017
Editor Note: The article is part of a newly launched ongoing series about immunooncology (IO) combination therapy, future articles of which will address PD-L1 and CTLA-4 combinations as well as IO in a curative setting, the Blueprint project, managing toxicities, as well as other related topics.
Immunotherapy, in particular blockade of cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) and the programmed cell death 1 pathway (PD-1/PD-L1), has been shown to be an effective treatment as monotherapy for some forms of cancer, especially lung cancer. Improved response rates and extended survival seen with immunotherapy have led investigators to explore the synergistic potential of combination immunotherapy to inhibit complementary immunosuppressive pathways simultaneously. With its established antitumor activity and favorable toxicity profile, PD-1/PD-L1 inhibition has served as the foundation for most combination immunotherapy strategies.
Rationale for Combination Immunotherapy
Combination immunotherapy aims to increase the percentage of patients who respond to treatment, to identify new tumor types that do not respond to monotherapy alone, and to improve the quality of clinical responses compared with monotherapy. Evidence suggests that PD-1 pathway inhibitors and other immune checkpoint inhibitors are most effective in tumors that are recognizable by the immune system. When tumors produce tumor antigens that are not sufficiently distinct from selfantigens, the tumor avoids detection by the immune system, and a spontaneous tumor response to treatment is absent. Combination strategies that involve complementary immunosuppressive pathways may enhance the tumor responses achieved with monotherapy and improve response rates in patients with lung cancer. One immunotherapy combination was recently approved and additional immunotherapy combinations are under investigation in patients with lung cancer.
Dual PD-1 Inhibition and Chemotherapy in Nonsquamous Non-Small Cell Lung Cancer
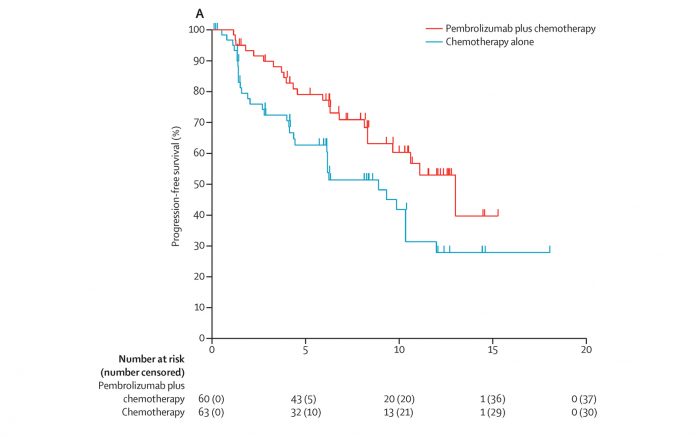
In May 2017, the U.S. Food and Drug Administration (FDA) approved pembrolizumab (Keytruda), an anti-PD-1 therapy, in combination with pemetrexed (Alimta) and carboplatin for the first-line treatment of metastatic nonsquamous non-small cell lung cancer (NSCLC), irrespective of PD-L1 expression. This approval was based on the results of the KEYNOTE-021 trial, cohort G.1 Updated results presented at the 2017 American Society of Clinical Oncology (ASCO) Annual Meeting showed that the frontline combination of pembrolizumab, pemetrexed, and carboplatin reduced the risk of progression or death by 50% and nearly doubled objective response rates (ORR) compared with chemotherapy alone.2 After 14.5 months of follow-up, the median progression-free survival (PFS) had not been reached in the triplet arm (95% CI, 8.5–not reached) compared with 8.9 months with chemotherapy alone (95% CI, 6.2–10.3). The 12-month PFS rate was 56% in the triplet arm compared with 34% with chemotherapy alone (HR, 0.50; 95% CI, 0.29–0.84; P = 0.0038). The ORR was 56.7% with pembrolizumab and 30.2% with chemotherapy alone (P = 0.0016). In addition, the hazard ratio for overall survival had dropped from 0.90 to 0.69, with a similar drop in the P value from 0.37 to 0.13, suggesting some further separation in outcomes favoring the pembrolizumab combination.
Dual PD-1/PD-L1 and EGFR Inhibition in Nonsquamous NSCLC
The phase Ib I4X-MC-JFCQ trial is investigating the combination of pembrolizumab and necitumumab (Portrazza), an epidermal growth factor receptor (EGFR) antibody, in patients with metastatic NSCLC who have received at least one prior line of therapy.3 At an interim analysis of 34 patients with nonsquamous NSCLC, the ORR was 29.4%. With a median follow-up of 6.0 months, the median PFS was 6.9 months, and the 6-month rate was 55.1%.
Dual PD-1/PD-L1 and CTLA-4 Inhibition in Small Cell Lung Cancer
Preclinical evidence has provided a strong rationale to investigate the combination of PD-1/PD-L1 and CTLA-4 inhibition in different tumor types.4 Dual blockade of PD-1/PD-L1 and CTLA-4 has proven effective in patients with advanced melanoma, and 2 studies are exploring this combination in patients with small cell lung cancer (SCLC).5,6
The phase I/II CheckMate 032 trial evaluated dual immunotherapy with nivolumab (Opdivo), a PD-1 inhibitor, and ipilimumab (Yervoy), a CTLA-4 immune checkpoint inhibitor, in 159 patients with recurrent SCLC.7 In the nonrandomized portion of the trial, 98 patients received nivolumab monotherapy and 61 patients received the combination. The ORR was 25% with the combination and 11% with monotherapy. Based on these initial results, a randomized cohort of 247 patients with SCLC was added. In the subsequent SCLC expansion cohort, patients were randomized 3:2 to nivolumab monotherapy or the combination and stratified by number of prior therapies. Preliminary efficacy data for this population were presented at the 2017 ASCO Annual Meeting. In the expansion cohort, the response rate to nivolumab plus ipilimumab was 21% compared with 12% with nivolumab monotherapy. These response rates were similar to those seen in the nonrandomized portion of the trial; however, longer follow-up is needed to validate these results.
Also underway is CheckMate 451, a randomized, phase III trial of nivolumab monotherapy or nivolumab plus ipilimumab as maintenance therapy in extensive- stage SCLC following first-line chemotherapy. Primary endpoints are overall survival and PFS. Results have not yet been reported.8
Dual PD-1/PD-L1 and Indoleamine 2,3-dioxygenase 1 (IDO1) Enzyme Inhibition
IDO1 is a key immunosuppressive enzyme that modulates the antitumor immune response by promoting regulatory T cell generation and blocking effector T cell activation, thereby facilitating tumor growth by allowing cancer cells to avoid immune surveillance. Epacadostat is a selective oral inhibitor of the IDO1 enzyme. The ongoing phase I/II ECHO- 202 trial is evaluating the combination of pembrolizumab and epacadostat in patients with advanced squamous and nonsquamous NSCLC.9 Patients previously treated with anti-PD-1 or anti- CTLA-4 therapies are excluded from this trial. Enrollment is complete for the phase I dose escalation and dose expansion portions of the trial. Preliminary results show an ORR of 35% with the combination, irrespective of PD-L1 status, for all patients combined.
Safety Concerns With Immunotherapy Combinations
Immunotherapies present distinct safety challenges, as immune responses may be raised against normal tissues as well as against tumor cells. Checkpoint inhibitors have been associated with several inflammatory conditions similar to autoimmune-like disorders, which may indicate a disruption of self-tolerance to normal tissues. Adverse events reported in patients treated with immunotherapies commonly involve certain organ systems, including the skin, endocrine organs, liver, gastrointestinal tract, nervous system, eyes, respiratory system, and hematopoietic cells.10 In addition to the synergistic therapeutic activity seen with immunotherapy combinations, there also may be substantive incremental toxicity, depending on the patient population and the dose and administration schedule employed. Standard dosing approaches may not work when combining immunotherapies, and patients need to be closely monitored so that safety concerns can be identified early and intervention delivered as soon as possible. By and large, steroids ameliorate these toxicities, but there are also concerns that steroids may potentially abrogate the positive effects of immunotherapy.
Clinical data thus far have shown the potential benefits that may be achieved with immunotherapy combinations. Nevertheless, further research is needed to better define the mechanisms of action of these combinations and to test various dosing schedules so that patients may benefit from these treatments. In addition, the risk-benefit profiles of immunotherapy combinations, as well as their economic impact, will need to be evaluated before novel combinations become standard of care. ✦
References
1. Langer CJ, Gadgeel SM, Borghaei H, et al. Carboplatin and pemetrexed with or without pembrolizumab for advanced, non-squamous non-small-cell lung cancer: a randomised, phase 2 cohort of the open-label KEYNOTE-021 study. Lancet Oncol. 2016;17:1497-1508. 2. Papadimitrakopoulou V, Gadgeel SM, Borghaei H, et al. First-line carboplatin and pemetrexed (CP) with or without pembrolizumab (pembro) for advanced nonsquamous NSCLC: updated results of KEYNOTE-021 cohort G. J Clin Oncol. 2017;35(suppl; abstr 9094).
3. Besse B, Garrido P, Puente J, et al. Efficacy and safety of necitumumab and pembrolizumab combination therapy in stage IV nonsquamous non-small cell lung cancer. 2016 World Conference on Lung Cancer. Abstract MA09.11. Presented December 6, 2016. 4. Curran MA, Montalvo W, Yagita H, Allison JP. PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors. Proc Natl Acad Sci USA. 2010;107(9):4275-4280. doi:10.1073/ pnas.0915174107.
5. Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373:23-34. doi:10.1056/NEJMoa1504030.
6. Postow MA, Chesney J, Pavlick AC, et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med. 2015;372:2006-2017. doi:10.1056/ NEJMoa1414428. 7. Hellmann MD, Ott PA, Zugazagoitia J, et al. Nivolumab (nivo) ± ipilimumab (ipi) in advanced small cell lung cancer (SCLC): first report of a randomized expansion cohort from CheckMate 032. J Clin Oncol. 2017;35(15 suppl):8503.
8. ClinicalTrials.gov [database]. An investigational immuno-therapy study of nivolumab, or nivolumab in combination with ipilimumab, or placebo in patients with extensive-stage disease small cell lung cancer (ED-SCLC) after completion of platinum-based chemotherapy (CheckMate 451). Last updated August 1, 2017. https://clinicaltrials.gov/show/NCT02538666. Accessed August 9, 2017.
9. Gangdahar TC, Schneider BJ, Bauer TM, et al. Efficacy and safety of epacadostat plus pembrolizumab treatment of NSCLC: preliminary phase I/II results of ECHO-202/ KEYNOTE-037. 2017 American Society of Clinical Oncology Annual Meeting. Abstract 9014. Presented June 3, 2017.
10. Amos SM, Duong CPM, Westwood JA, et al. Autoimmunity associated with immunotherapy of cancer. Blood. 2011;118:499-509.










