By Heather Wakelee, MD
Posted: October 2017
For most of the world, four cycles of cisplatin-based adjuvant chemotherapy is the standard of care for patients with resected stage II and IIIA non-small cell lung cancer (NSCLC), and is offered to many patients with stage IB tumors at least 4 cm in size or larger. This approach provides only a modest survival benefit with meta-analyses revealing a 4–5% absolute survival benefit at 5 years with adjuvant chemotherapy.1,2 Attempts to improve outcomes with the addition of other agents to cisplatin doublets have been disappointing, including the negative ECOG-ACRIN E1505 adjuvant trial with bevacizumab3 and the MAGRIT trial with the MAGE-A3 vaccine.4 In subsets of patients with known driver mutations, the use of targeted agents in the adjuvant setting is an area of active investigation.
In metastatic NSCLC, epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs) produce superior response and progression-free survival (PFS) compared with platinum doublet chemotherapy for patients with tumors with activating EGFR mutations (EGFRmut) such as exon 19 deletion (del19) and the exon 21 mutation L858R.5,6
Retrospective data and non-randomized trials of adjuvant EGFR TKIs have been promising, but limited. The first phase III adjuvant EGFR TKI trial was RADIANT; however, patients were selected for EGFR expression by IHC/FISH and not by EGFR mutation status.7 The primary endpoint was disease- free survival (DFS) in the full data set of patients randomized to erlotinib versus placebo after completion of any planned adjuvant chemotherapy, with secondary analyses focused on patients with del19 or L858R EGFR mutations in their tumor. In the entire study population, no differences were found in either DFS or overall survival (OS), but in the EGFRmut subset (N=161) DFS favored erlotinib (HR 0.61, 95% CI = 0.384– 0.981, P= 0.0391). Based on trial statistical design this DFS benefit was not statistically significant. No OS benefit was reported for the EGFRmut subset, though OS results remain immature. Most concluded from RADIANT that adjuvant EGFR TKI was not a standard treatment option, and further investigation was warranted.
ADJUVANT (CTONG 1104) is the first randomized phase III trial focused exclusively in patients with resected EGFR mutant (+) NSCLC. Dr. Yi-Long Wu gave a wonderful presentation of the data at ASCO 2017. The study was restricted to patients with resected stage II-IIIA (N1-N2) EGFR mutation (+) NSCLC. The patients were evenly split between del19 and L858R mutations, and the majority underwent a lobectomy (82% and 84% on the chemotherapy and gefitinib arms, respectively). Prognostic factors were well balanced, but it is important to note that nearly two-thirds of the patients (>64%) had N2 disease. The study included 220 patients who were randomized 1:1 to gefitinib at 250 mg daily for 24 months or to cisplatin (75 mg/m2 day 1) plus vinorelbine (25 mg/m2 day 1,8) every 3 weeks for up to 4 cycles. DFS was the primary endpoint. Of 483 patients assessed for eligibility, 222 patients were evenly randomized with 111 assigned to each arm, but the treatment refusal rate was much higher on the chemotherapy arm with only 87 (78%) receiving chemotherapy while 106 (95%) received assigned gefitinib. Thus of the 222 patients treated, of whom 64% had stage IIIA disease, only 39% of them received any chemotherapy. The majority of patients who initiated therapy completed treatment with 84% of the 87 who received chemotherapy completing 4 cycles and 68% of those on gefitinib completing at least 18 months of therapy.
The study met its primary endpoint: median DFS was 28.7 months for gefitinib versus 18.0 months with chemotherapy (HR for recurrence 0.60, 95% CI 0.42–0.87, p.005). However, there was no clear “tail” as the vast majority of patients had recurrent disease by 48 months and the 3-year DFS was only 34% with gefitinib versus 27% on the chemotherapy arm. At first glance these results are very impressive. However, one must remember that the majority of patients on this study had stage IIIA disease, and many of these patients never received any chemotherapy. It is noteworthy that the forest plot revealed that patients with N1 disease did not have as favorable a benefit with gefitinib with a HR of 0.89 (95% CI 0.45–1.76, p.743); the most significant benefit was in the N2 nodal group with a HR of 0.52 (95% CI 0.34–0.80, p.003). Many of the N2 patients likely had more extensive disease. Toxicity was as expected with higher rates of grade 3 events with chemotherapy, mostly hematologic, though it is noteworthy that the chemotherapy duration was only 4 cycles (approximately 3 months) versus 2 years for gefitinib. Not surprisingly, health-related quality of life favored gefitinib. The OS data were immature and not presented. The conclusion was that “adjuvant gefitinib could be the preferred approach in patients with resected N1/N2 EGFRmutant NSCLC.” The question though is really about what happens after recurrence. By year 3, a significant majority of patients had recurred. Presumably, those on the chemotherapy arm would then go on to receive an EGFR TKI, while those on gefitinib might or might not receive chemotherapy. Hence, the survival outcomes based on these approaches will be critical to determining the best possible strategy. If the gefitinib was merely treating undetected metastatic disease, the superior DFS versus chemotherapy is not surprising as PFS superiority for EGFR TKIs versus chemotherapy in the metastatic setting is well established. The real question for an adjuvant trial is whether or not the intervention actually impacts cure rates and survival. For that answer we need longer follow-up from this trial.
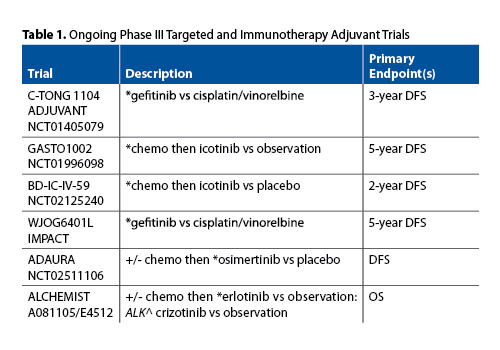
ADJUVANT (CTONG 1104) is not the only adjuvant EGFR TKI study proceeding globally. Multiple others are listed in Table 1. The WJOB6401L study in Japan is the most similar to ADJUVANT, with a nearly identical design; the outcome will clearly influence interpretation of the ADJUVANT (CTONG 1104) results. Other trials, including many with the EGFR TKI icotinib in China, are not only looking at whether an OS benefit can be obtained with adjuvant molecularly targeted therapy but are also assessing the duration of therapy and the potential to use EGFR TKIs instead of or after chemotherapy in selected patients. The largest North American study is the US NCI National Clinical Trials Network (NCTN) ALCHEMIST trial. The study screens patients with resected early stage (IB-IIIA) NSCLC for EGFR-activating mutations and ALK translocations. If either is identified, then, after completion of all planned adjuvant chemotherapy or radiation therapy, patients are randomized to targeted TKI therapy for 2 years or to observation. Both sub-studies are expected to enroll approximately 400 patients and are powered for an OS endpoint. The results of these trials will more clearly establish the role, if any, for adjuvant TKI therapy.
Does ADJUVANT provide enough data to support the conclusion “adjuvant gefitinib could be the preferred approach in patients with resected N1/N2 EGFRmutant NSCLC”? Personally, I believe that until the OS results show a clear benefit to support this approach, we should not move away from the proven benefits of chemotherapy. However, in the setting where a patient refuses chemotherapy, the ADJUVANT data strongly support that EGFR TKI treatment is better than no treatment. Given the preponderance of stage III patients in ADJUVANT, and lack of benefit in N1 patients in the forest plot from the trial, it is not clear how this will translate to patients diagnosed at earlier stages. We must exercise caution in how this study is interpreted and presented to patients. ADJUVANT clearly demonstrates that for patients with resected EGFRmut NSCLC with N2 involvement, gefitinib is an appropriate therapy, but given the extremely high rates of recurrence, it is not clear that this is truly adjuvant therapy, versus early initiation of treatment for occult metastatic disease. We must also not forget that nearly a quarter of patients assigned to the chemotherapy arm (22%) refused treatment and thus were treated with surgery alone, clearly an inferior strategy for stage IIIA NSCLC.
ADJUVANT (CTONG 1104) is an important, well-conducted study of adjuvant gefitinib in patients selected for resected EGFR mutation (+) NSCLC, and the first of this class of studies to be completed. The authors are to be congratulated on the presentation of this important trial. However, except in the subset of patients with resected EGFR mut (+) N2 disease who refuse chemotherapy, I believe we should wait before making adjuvant gefitinib a standard approach. We need to see a clear OS benefit, and we need to see the results of the many other ongoing trials, before changing global practice. ✦
References
1. Pignon JP, Tribodet H, Scagliotti GV, et al: Lung Adjuvant Cisplatin Evaluation: A Pooled Analysis by the LACE Collaborative Group. J Clin Oncol. 2008;26:3552-3559.
2. Group NM-aC, Arriagada R, Auperin A, et al: Adjuvant chemotherapy, with or without postoperative radiotherapy, in operable non-smallcell lung cancer: two meta-analyses of individual patient data. Lancet. 2010;375:1267-1277.
3. Wakelee HA, Dahlberg SE, Keller SM, et al: Randomized phase III trial of adjuvant chemotherapy with or without bevacizumab in resected nonsmall cell lung cancer (NSCLC): Results of E1505. J Thorac Oncol Proceedings WCLC 2015:Abstr: Plen04.03, 2015.
4. Vansteenkiste JF, Cho BC, Vanakesa T, et al: Efficacy of the MAGE-A3 cancer immunotherapeutic as adjuvant therapy in patients with resected MAGEA3- positive non-small-cell lung cancer (MAGRIT): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2016; 17:822-835.
5. Mok TS, Wu YL, Thongprasert S, et al: Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361:947-957.
6. Sequist LV, Yang JC, Yamamoto N, et al: Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol. 2013;31: 3327-3334.
7. Kelly K, Altorki NK, Eberhardt WE, et al: Adjuvant erlotinib versus placebo in patients with stage IB-IIIA non-small-cell lung cancer (RADIANT): A randomized, double-blind, phase III trial. J Clin Oncol. 2015;33:4007-4014.











