By Cynthia L. Kryder, MS, CCC-Sp
Editor Note: IASLC Lung Cancer News is pleased to provide the following overview, which is followed by expert commentary by Dr. Heather Wakelee, Dr. Yi-Long Wu, and Dr. Julien Mazières.
In patients with resected stage II-IIIA non-small cell lung cancer (NSCLC), cisplatin-based adjuvant chemotherapy over the last decade has become the standard of care, based on clinical trials that have demonstrated a statistically significant survival benefit in patients with completely resected stage IB, II, or III NSCLC.1 Results from a recent clinical trial suggest that targeted therapy with the epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor gefitinib (Iressa) may be a better option than chemotherapy to improve disease-free survival (DFS) in patients with sensitizing EGFR mutations.2
In advanced NSCLC, the presence of EGFR exon 19 deletions or exon 21 (L858R) substitution mutations is predictive of treatment benefit with an EGFR tyrosine kinase inhibitor with multiple trials showing a statistically significant and clinically meaningful response and PFS benefit compared to standard chemotherapy. Consequently, these mutations are referred to as sensitizing EGFR mutations. About 10% to 15% of Caucasian patients with NSCLC and up to 50% of Asian patients have sensitizing EGFR mutations.3 Accordingly, experts recommend testing for EGFRsensitizing mutations in all patients with nonsquamous NSCLC or NSCLC not otherwise specified (NOS).4
Gefitinib is an oral tyrosine kinase inhibitor that is well established in Asia for the treatment of advanced NSCLC. It was reapproved in the United States in July 2015 for first-line treatment of patients with metastatic NSCLC whose tumors have EGFR exon 19 deletions or exon 21 (L858R) substitution mutations. This approval was based on the results of a phase IV, open-label, single-arm trial.5 At the Annual Meeting of the American Society for Clinical Oncology (ASCO) in June 2017, Yi-Long Wu, MD, of the Guangdong General Hospital, in China, presented new data from the randomized ADJUVANT trial that suggest that gefitinib might play a significant role in the adjuvant setting, as well.
The ADJUVANT Trial
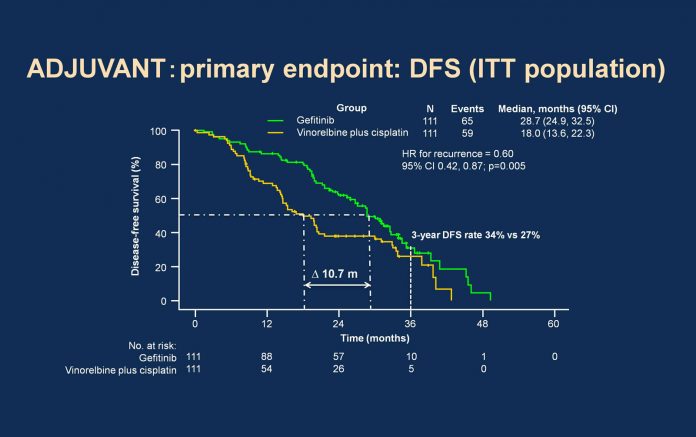
The phase III ADJUVANT (Chinese Thoracic Oncology Group 1104) trial was the first randomized trial to compare gefitinib head-to-head with vinorelbine plus cisplatin in 222 patients with completely resected stage II-IIIA (N1-N2) NSCLC with confirmed EGFR-activating mutations.2 Patients received gefitinib 250 mg once daily for 24 months or the vinorelbine-cisplatin combination every 3 weeks for four cycles. Patients were stratified according to lymph node status and EGFR-mutation status. The primary endpoint was DFS in the intentto- treat population. Secondary endpoints included 3-year DFS, 5-year DFS, overall survival (OS), 5-year OS, safety, healthrelated quality of life, and exploratory biomarker analyses. At a median follow-up of 36.5 months (range 0.1 to 62.8 months), patients who received gefitinib had significantly longer DFS than those receiving chemotherapy. Median DFS was 28.7 months (95% confidence interval [CI] 24.9 to 32.5) for gefitinib compared with 18.0 months for vinorelbine plus cisplatin (95% CI 13.6 to 22.3; hazard ratio 0.60; 95% CI 0.42 to 0.87; P=0.005). Significantly more patients in the gefitinib arm were disease-free at 3 years compared with the chemotherapy arm, 34% versus 27%, respectively (P=0.013). The number of overall survival events was 76 (34.2%); consequently, the survival data were immature for assessment. A significant correlation between lymph node status (pN1/N2) and DFS (P<0.05) was seen in a subgroup analysis of patients in the gefitinib arm.
With regard to adverse events (AEs), patients treated with gefitinib had fewer grade 3 or higher AEs than those in the chemotherapy arm (12.3% vs 48.3%; P<0.001). All-grade AEs occurred in 57.5% of patients treated with gefitinib, compared with 80.5% of the chemotherapy group. Hematologic AEs, nausea, vomiting, and anorexia were more frequent with chemotherapy; however, rash, elevated liver enzymes, and diarrhea occurred more often with gefitinib. No gefitinib-treated patients developed interstitial lung disease.
Implications for Practice
The results of the ADJUVANT trial show that a subset of patients with resected stage II-IIIA (N1-N2) NSCLC can benefit from targeted treatment that has fewer side effects than chemotherapy. In the US, where it is not standard practice to perform EGFR mutation testing immediately after surgery, these data point to the potential benefit of testing tumors immediately after surgery rather than waiting until cancer recurs or metastasizes to determine whether treatment with an EGFR inhibitor can be initiated in earlier-stage disease.
Nevertheless, whether practice patterns will change as a result of these data is open for debate. A key unanswered question is what effect will adjuvant gefitinib have on overall survival? Dr. Wu and colleagues will continue following the patients in the ADJUVANT trial to fully measure this key parameter. Others question the omission of standard chemotherapy in the investigational arm prior to the administration of gefitinib.
Additional factors that may impact clinical practice are treatment length and cost. In the ADJUVANT trial, patients in the gefitinib arm received treatment for 2 years, compared with 12 weeks for patients in the chemotherapy cohort. Longer treatment may be a burden to patients and could lead to decreased treatment compliance as well as cumulative toxicities. In this regard, a TWIST analysis in each arm measuring time without progression as well as toxicities of treatment could prove instructive. In addition, as a specialty pharmaceutical, gefitinib costs more than an average outpatient drug and certainly more than chemotherapy; its estimated wholesale cost is approximately US$7,000 per month.6
Although adjuvant gefitinib was less toxic and more effective than chemotherapy in preventing recurrence following surgery in patients with sensitizing EGFR mutations, practitioners await the results of ongoing and future clinical trials to determine the optimal role of gefitinib in the treatment of NSCLC. Based on National Comprehensive Cancer Network guidelines, it has not yet entered standard practice in the US. ✦
References
1. Arriagada R, Bergman B, Dunant A, et al. Cisplatin-based adjuvant chemotherapy in patients with completely resected non-small cell lung cancer. N Engl J Med. 2004;350:351-360.
2. Wu Y-L, Zhong W, Wang Q, et al. Gefitinib (G) versus vinorelbine+cisplatin (VP) as adjuvant treatment in stage II-IIIA (N1-N2) non-smallcell lung cancer (NSCLC) with EGFR-activating mutation (ADJUVANT): a randomized, phase III trial (CTONG 1104). J Clin Oncol. 2017 (suppl; abstr 8500). http://abstracts.asco.org/199/ AbstView_199_188666.html. Accessed June 26, 2017.
3. Hirsch FR, Bunn PA Jr. EGFR testing in lung cancer is ready for prime time. Lancet Oncol. 2009;10:432-433.
4. National Comprehensive Cancer Network. NCCN Guidelines Version 7.2017 Non-small cell lung cancer. Revised June 22, 2017. https://www.nccn. org/professionals/physician_gls/pdf/nscl.pdf. Accessed June 26, 2017.
5. Douillard JY, Ostoros G, Cobo M, et al. First-line gefitinib in Caucasian EGFR mutation-positive NSCLC patients: a phase IV, open-label, singlearm study. Br J Cancer. 2014;110:55-62.
6. Million RP. Drug pricing: oncology in the United States [white paper]. Trinity Partners LLC. June, 2016. http://www.trinitypartners.com/ files/2314/6498/9546/Trinity_Partners_White_ Paper_-_Drug_Pricing_in_Oncology_-_Ryan_P._ Million_-_June_2016.pdf. Accessed June 26, 2017.