By Cynthia L. Kryder, MS, CCC-Sp
Rearrangements in the anaplastic lymphoma kinase (ALK) gene occur in approximately 2% to 7% of patients with advanced non-small cell lung cancer (NSCLC).1 For patients with advanced ALK-positive NSCLC, the current firstline standard of care is crizotinib, a MET tyrosine kinase inhibitor (TKI) with activity in ALK-rearranged NSCLC. It has been shown to yield very high response rates (exceeding 60%) and to improve progression-free survival (PFS) compared with standard chemotherapy when used in patients with advanced ALK-positive NSCLC whose disease has progressed on previous chemotherapy, including those with brain metastases. 2-4 Nevertheless, most patients with ALK-positive NSCLC treated with firstline crizotinib will eventually relapse, either due to the development of ALK resistance mutations or inadequate CNS drug penetration.3,5 Ceritinib, alectinib, and brigatinib are next-generation ALK TKIs that have emerged as standard therapy for patients with advanced ALK-positive NSCLC who experience disease progression while on crizotinib. These agents have been shown to be more potent with more brain penetrance than crizotinib. Additionally, ceritinib and alectinib both demonstrate activity against common crizotinib-resistance mutations, such as the gatekeeper ALK L1196M mutation.6,7
Emerging data suggest that these agents also may have a role in the first-line setting. The global, randomized phase III ASCEND-4 trial compared ceritinib with platinum/pemetrexed chemotherapy in newly diagnosed patients with advanced ALK-positive NSCLC. Ceritinib reduced the risk of disease progression or death by 45% compared with standard chemotherapy. Patients who received ceritinib had significantly longer median PFS, 16.6 months versus 8.1 months in the chemotherapy arm (HR = 0.55, P<0.00001).8 Based on these positive results, ceritinib (Zykadia) was approved by the FDA on May 26, 2017, for first-line treatment of patients with ALK-positive, advanced NSCLC.
Following closely behind is alectinib, which in the phase III global ALEX trial showed superior efficacy compared with crizotinib as first-line therapy for treatment-naive patients with advanced ALK-positive NSCLC. Compared with crizotinib, alectinib prolonged PFS, as well as the time to CNS progression, in treatment-naive patients with ALKpositive NSCLC. Results of the ALEX trial were presented in June at the 2017 annual meeting of the American Society of Clinical Oncology during the thoracic oncology plenary session.9 The ALEX Trial The open-label, randomized phase III ALEX trial compared first-line alectinib 600 mg BID with crizotinib 250 mg BID in patients with stage IIIB/IV ALK-positive NSCLC, as determined by immunohistochemistry. The trial enrolled 303 systemic-treatment-naive patients from 29 countries. About 40% of patients in each treatment arm had CNS metastases, all of whom had undergone CNS-metastasis treatment, including whole-brain radiotherapy, radiosurgery, brain surgery, or a combination of these modalities.
The primary endpoint was investigator- assessed PFS, with systematic CNS imaging in all patients. Secondary endpoints included independent review committee (IRC)-assessed PFS, IRC-assessed time to CNS progression (TTP), objective response rate (ORR), overall survival (OS), and safety.
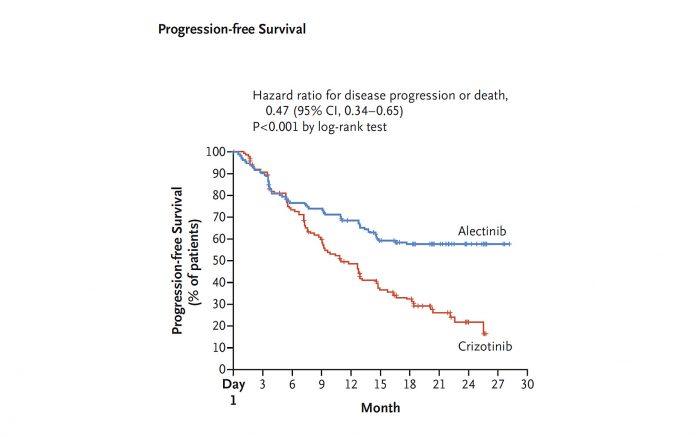
Patients who received alectinib experienced significantly longer PFS than those who received crizotinib (HR 0.47, 95% CI 0.34–0.65, P<0.0001). Median PFS was 11.1 months (95% CI 9.1–13.1) for crizotinib, but was not reached in the alectinib arm (95% CI 17.7–not estimated [NE]). Overall survival data were not yet mature.
Time to CNS progression was a key secondary endpoint of this trial. Alectinib showed neuroprotective capacity and significantly delayed CNS progression. CNS progression was detected in 12% of patients in the alectinib arm, compared with 45% in the crizotinib arm (HR 0.16, P<0.0001). The cumulative incidence rates of 12-month CNS progression were 9.4% (95% CI 5.4–14.7) in the alectinib arm and 41.4% (95% CI 33.2–49.4) in the crizotinib arm.
Other key secondary endpoints showed superiority for alectinib compared with crizotinib. IRC-assessed median PFS was 25.7 months (95% CI 19.9–NE) for alectinib versus 10.4 months (95% CI 7.7–14.6) for crizotinib. The IRC-assessed cause-specific hazard ratio of CNS progression was 0.16 (95% CI 0.10–0.28, P<0.0001). The ORR was 83% (95% CI 76–89) for alectinib versus 76% (95% CI 68–82, P=0.09) for crizotinib.
With regard to adverse events (AEs), patients treated with alectinib had fewer grade 3 and 4 AEs, 41% versus 50% with crizotinib. The rate of AEs leading to discontinuation, dose reduction, and interruption of treatment was lower in the alectinib arm.
The results of the ALEX trial replicate earlier findings of J-ALEX, a phase III trial that compared alectinib (300 mg BID) and crizotinib head to head in 207 Japanese patients with ALK-positive NSCLC.10 At the second interim analysis, median PFS had not yet been reached with alectinib (95% CI 20.3-NE) and was 10.2 months (95% CI 8.2-12.0) with crizotinib.
Where Do We Go Next?
The results of the ASCEND-4 and the ALEX trials signal a shifting treatment paradigm that moves away from cytotoxic chemotherapy and crizotinib toward next-generation ALK inhibitors as first-line therapy for patients with advanced ALK-positive NSCLC. Still, several questions remain. For example, given the neuroprotective activity of alectinib, what is the role of radiotherapy in patients who present with CNS metastases at the time of diagnosis? And how should physicians treat patients who develop resistance to first-line ceritinib and alectinib? Can we develop a single algorithm based on resistance mutations that occur on treatment to determine which TKI to administer next? Or do we default to standard chemotherapy once second-line agents migrate into the firstline setting? Ultimately, a better understanding of the resistance mechanisms will be necessary to develop future effective second-line therapies.
Still unknown is the effect that nextgeneration ALK inhibitors will have on overall survival. Whether the prolonged PFS seen with alectinib and ceritinib translates into a survival advantage has not yet been determined. Nevertheless, the results of the ALEX trial point to a new standard of care for previously untreated patients with ALK-positive NSCLC. ✦
References
1. Kwak EL, Bang YJ, Camidge DR, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med. 2010;363:1693-1703.
2. Solomon BJ, Mok T, Kim DW, et al. First-line crizotinib versus chemtherapy in ALK-positive lung cancer. N Engl J Med. 2014;371:2167-2177.
3. Costa DB, Shaw AT, Ou SH, et al. Clinical experience with crizotinib in patients with advanced ALKrearranged non-small-cell lung cancer and brain metastases. J Clin Oncol. 2015;33:1881-1888.
4. Shaw AT, Yeap BY, Solomon BJ, et al. Impact of crizotinib on survival in patients with advanced, ALK-positive NSCLC compared with historical controls [abstract]. J Clin Oncol. 2011;29(Suppl 15):Abstract 7507.
5. Zhang I, Zaorsky NG, Palmer JD, et al. Targeting brain metastases in ALK-rearranged non-small-cell lung cancer. Lancet Oncol. 2015;16:e510-e521.
6. Friboulet L, Li N, Katayama R, et al. The ALK inhibitor ceritinib overcomes crizotinib resistance in non-small cell lung cancer. Cancer Discov. 2014;4:662-673.
7. Katayama R, Khan TM, Benes C, et al. Therapeutic strategies to overcome crizotinib resistance in non-small cell lung cancers harboring the fusion oncogene EML4-ALK. Proc Natl Acad Sci USA. 2011;108:7535-7540.
8. Soria JC, Tan DS, Chiari R, et al. First-line ceritinib versus platinum-based chemotherapy in advanced ALK-rearranged non-small-cell lung cancer (ASCEND-4): a randomised, open-label, phase 3 study. Lancet. 2017;389:917-929.
9. Shaw AT, Peters S, Mok T, et al. Alectinib versus crizotinib in treatment-naive advanced ALKpositive non-small cell lung cancer (NSCLC): primary results of the global phase III ALEX study. 2017 ASCO Annual Meeting. Abstract LBA9008. Presented June 6, 2017. http://meetinglibrary.asco. org/record/153629/abstract. Accessed June 20, 2017. 10. Hida T, Nokihara H, Kondo M, et al. Alectinib versus crizotinib in patients with ALK-positive non-small cell lung cancer (J-ALEX): an open-label, randomised phase 3 trial. Lancet. 2017 May 10. pii: S0140-6736(17)30565-2. doi: 10.1016/S0140- 6736(17)30565-2. [Epub ahead of print].










