Posted: February 2017
By Fred R. Hirsch, MD, PhD
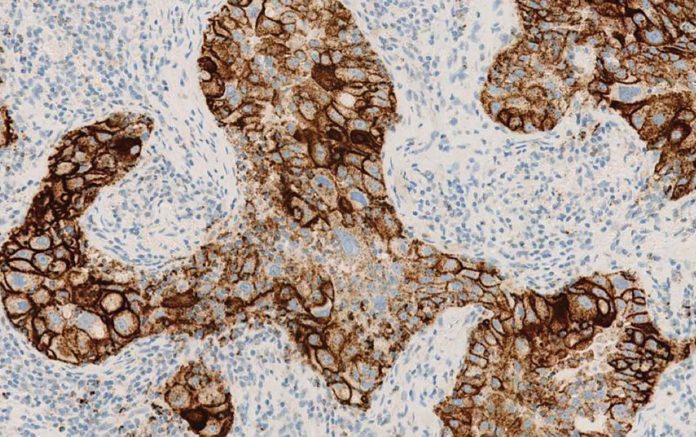
In the new era of immunotherapy in thoracic cancer, evaluating PD-L1 status remains a clinical challenge. In recognition of the potential confusion generated by having multiple predictive assays related to the same “family” of drugs, a workshop led by the U.S. Food and Drug Administration (FDA), the American Association for Cancer Research (AACR), and the American Society of Clinical Oncology (ASCO) was held in 2014 to discuss this matter. Several pharmaceutical and diagnostic companies with interest in the field as well as other academic organizations participated in this workshop. As a result of the workshop, the PD-L1 Blueprint Project was established, with the primary goal of comparing the PD-L1 assays used in clinical trials in terms of analytical and diagnostic performance. The consortium behind the Blueprint Project included representatives from Bristol-Myers Squibb (BMS), Merck, Genentech/Roche, AstraZeneca, Dako, Ventana, and AACR, as well as the International Association for the Study of Lung Cancer (IASLC), which is coordinating the project.
The first phase of the project, a feasibility study of a relative small number of cases (38), has just been published in the Journal of Thoracic Oncology.1 The study showed that 3 of 4 assays (Dako’s 28-8 linked to nivolumab, Dako’s 22C3 linked to pembrolizumab, and Ventana’s SP 263 linked to durvalumab) were very similar in analytical performance for PD-L1 expression on tumor cells, while the fourth assay, Ventana’s SP-142, linked to atezolizumab, consistently showed PD-L1 expression in fewer tumor cells compared to the other three assays. All assays expressed PD-L1 on immune cells, but with much greater variation compared to tumor cell expression. With respect to diagnostic comparability using selected cutoff values for comparison, this study demonstrated that despite using the same cutoff values, some patients will be categorized as PD-L1 positive by one assay, but negative by another, strongly suggesting that these assays are not automatically interchangeable. However, as this study focused on feasibility and included a small number of selected tumors, no firm conclusions on the interchangeability among assays can be drawn. Therefore, a much larger ongoing phase II study is currently attempting to address this clinically important question and to compare performance of assays between large tumor specimens, smaller biopsies, and cytology.
The major strength of the PD-L1 Blueprint Project is the unique partnership among the different pharmaceutical companies and diagnostic companies, with IASLC as the coordinating organization.
The major strength of the PD-L1 Blueprint Project is the unique partnership among the different pharmaceutical companies and diagnostic companies, with IASLC as the coordinating organization. More recently, Pfizer/Merck Serono has been added to the consortium since this company is conducting clinical trials with avalumab. Furthermore, the Blueprint Project is the only study to compare all the clinical PD-L1 assays as they are used in the clinical trials; three of these are FDA approved for clinical practice (Dako’s 22C3 and 28-8, and Ventana’s SP-142).
Currently, pembrolizumab (Merck & Co.) is approved with a “companion diagnostic,” e.g., 22C3 required for use of the drug, while the two other assays (28-8 and SP-142) are not required for use of their respective drugs, e.g., nivolumab and atezolizumab, but approved as “complimentary assays.”
The approval and clinical development of PD-L1 assays not only hinges on the different antibodies used, but also the interpretation of different staining platforms specific to each of the assays. As currently constituted, there is a high risk of random matching of antibodies, staining platforms, and drug selection, which might yield results that do not have clinical validity. The phase II component of the Blueprint Project is anticipated to give a much better understanding of similarities and differences between the assays and their clinical interchangeability.
Reference
1. Hirsch FR, McElhinny A, Stanforth D, et al. PD-L1 Immunohistochemistry Assays for Lung Cancer: Results from Phase 1 of the Blueprint PD-L1 IHC Assay Comparison Project. J Thorac Oncol. 2017;12:208-222.