Posted: February 2017
By Lori Alexander, MTPW, ELS, MWC
Drug development has become a biomarker- driven process, necessitating new approaches and clinical trial designs. Speakers addressed these issues in the WCLC session “Anticancer Drug Development in the 21st Century.”
Former IASLC President Tony Mok, MD, of Chinese University of Hong Kong, explained that the two most important lessons learned from the development of EGFR tyrosine kinase inhibitors are related to biomarkers and endpoints. He detailed the history of gefitinib and erlotinib, noting the positive findings in early-phase studies but inconsistent outcomes in later studies of unselected study populations. The US Food and Drug Administration (FDA) approved both gefitinib and erlotinib (in 2003 and 2004, respectively) as second-line or third-line treatment of locally advanced or metastatic NSCLC, but the FDA withdrew its approval of gefitinib in 2005 when subsequent randomized phase III studies failed to show a survival benefit. Researchers then sought to determine clinical and tumor-related characteristics predictive of response to EGFR inhibitors. “We were desperate for a clinically relevant biomarker as the available biomarkers (IHC and FISH) were not helpful in patient selection,” said Dr. Mok. The primacy of EGFR mutation ultimately emerged. He said that it is essential to know the expected efficacy of a new agent in a biomarker-selected population and the incidence of the biomarker in the study population. An interaction test should confirm the predictive power. With regard to endpoints, Dr. Mok said that overall survival should be avoided as a primary endpoint when significant crossover is expected in the biomarkerselected population.
Charles Rudin, MD, PhD, of Memorial Sloan-Kettering Cancer Center, New York, US, asked the question “Is molecular-based therapy for lung cancer the way forward?” and promptly answered “Yes.” However, he followed up with another question: “But how do we find the right target in the right context?” He said that driver mutations must be found and then targeted, and that researchers need to work backward by starting with a clinical problem and figuring out why it fails. Finding some targets is more difficult, he added, and many may be hiding in regulation of the epigenome. Dr. Rudin also noted that resistance to targeted therapy must be addressed.
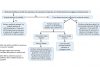
Yu Shyr, PhD, of Vanderbilt University School of Medicine, Nashville, US, provided an overview of drugs recently approved by the FDA for lung cancer, noting that the indications have often become specific to the results of molecular testing. He then discussed several biomarker-based trial designs, including enrichment, stratified, hybrid, umbrella, and basket designs. With the enrichment design, the biomarker is assessed and patients who test negatively for the biomarker are excluded; patients who test positively for the biomarker are then randomly assigned to treatment groups. The stratified design involves stratifying participants according to biomarker status and then randomly assigning to treatment. With a hybrid design, only certain biomarker subgroups are randomly assigned, while others are assigned to the standard of care. Dr. Shyr noted that, with an umbrella trial design, within one tumor type or histology, patients are grouped based on biomarker status to different randomizations (targeted treatments); with a basket trial design, patients are grouped based on biomarker status to identical treatment regardless of the tumor type or histology.
Francesco Pignatti, MD, of the European Medicines Agency (EMA), discussed the challenges related to the identification of small subgroups of molecularly defined patients. Given this situation, he said that it may be difficult to generate sufficient efficacy and safety data to assess benefit and risk, and that randomized controlled trials may not be feasible. Dr. Pignatti said that when assessing evidence from clinical trial data in small populations, researchers must use all available evidence, i.e., from small randomized controlled trials, multiple endpoints, multiple trials and sources, and external controls.
Dr. Pignatti also discussed greater involvement of patients when weighing benefits and risks. Some patients may be willing to take on higher risks to potentially achieve a small benefit. “If a significant group of reasonable and well-informed patients accepts this sort of trade-off, this may support a favorable benefit-risk profile,” he said. He also described Medicines Adaptive Pathways for Patients (MAPPs), a prospectively planned, adaptive approach to bringing drugs to market and further development. MAPPs balance timely access with providing adequate evolving information on benefits and harms, he said, and increase dialogue with stakeholders through the development process.