By Naiyer A. Rizvi
The rapid development of anti-PD-1 and PD-L1 antibodies in cancer underscores the tremendous impact they have had on the cancer landscape and for our patients. The first publication in 2010 forever changed our notion of “immunogenic” and “non-immunogenic” tumors.1 In this landmark paper, nivolumab, an immunotherapy agent, demonstrated activity not just in melanoma, but tumor regression was also observed in a patient with non-small cell lung cancer (NSCLC). Furthermore, tumor cell surface expression of B7-H1 (PD-L1) showed a correlation with the likelihood of response to treatment.
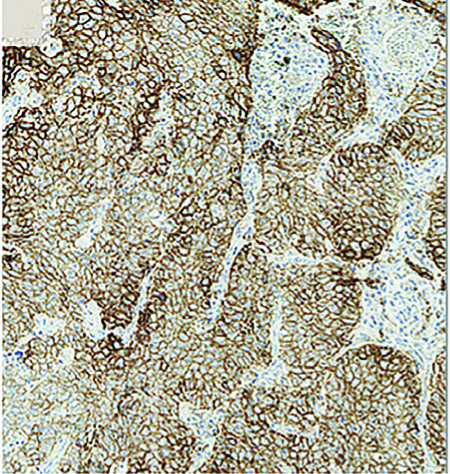
Fast-forward to 2015 and nivolumab has now been approved in the US for second-line therapy for both squamous and non-squamous lung cancer independent of PD-L1 expression.2,3 Also in 2015, pembrolizumab initially received accelerated approval for PD-L1-positive NSCLC4,5; and, as of October 24, 2016, it was approved in the first-line, treatmentnaive NSCLC setting for individuals with PD-L1 expression ≥50% and in the secondline setting in those with PD-L1 scores of 1% or higher; Figure 1 displays high levels of PD-L1 expression on a tumor.
Herein lies the controversy of PD-L1 as a biomarker for response to NSCLC. Although it is (mostly) clear that tumoral PD-L1 expression improves the likelihood of response to anti-PD-1 antibodies, many questions remain as to the optimal assay and cutoff. This controversy has proven less relevant in the second-line NSCLC setting where standard chemotherapy has limited efficacy and moderate toxicity; however, one must ask if measuring PD-L1 expression can help anti-PD-1 antibodies selectively displace chemotherapy in the first-line chemotherapy- naive setting.
Given the higher efficacy of chemotherapy in the first-line setting, PD-L1 expression has become relevant as a biomarker; two phase 3 trials comparing anti-PD-1 vs. first-line chemotherapy were presented at ESMO 2016 to address this very question. KEYNOTE 24 (KN 24)6 compared pembrolizumab with first-line chemotherapy and CheckMate 26 (CM 26) compared nivolumab with first-line chemotherapy. The key difference between the two trials was the cutoff to define positivity; KN 24 employed a cutoff of 50% PD-L1 expression (prevalence of 30%) and CM 26 employed a cutoff of 5% (50% prevalence); both were designed to demonstrate superiority in progression-free survival (PFS) with anti- PD-1 therapy. CM 26 failed to meet its primary endpoint; however, KN 24 demonstrated superiority for pembrolizumab compared to standard chemotherapy for both PFS and overall survival (OS). This remarkably positive trial demonstrated a response rate of 45% for pembrolizumab compared to 28% with chemotherapy, including a nearly 10% complete response rate. PFS was 10.3 vs. 6 months and 1-year landmark survival was 70 vs. 54%. These differences were statistically significant. The high response rate and complete response rate in KN 24 raise the question as to the potential greater efficacy of immunotherapy in the first-line setting. Does immunotherapy work better firstline versus second-line in NSCLC? Does prior chemotherapy impede the magnitude of immune response in some way?
These data have opened the door for broader immunotherapy indications in the first-line setting beyond the high positive PD-L1 subset. As we look to the future NSCLC landscape, trials combining chemotherapy with anti-PD-(L)1 antibodies are underway, as are trials combining anti- CTLA-4 with anti- PD-(L)1 antibodies. These trials may help us extend the application of immunotherapy in the first-line setting beyond the high PD-L1 positive patients; the results are eagerly awaited.
Questions certainly remain as to whether immunotherapy can be applied to every patient with NSCLC. KN 24 inclusion criteria included ECOG 0-1, no corticosteroid use, and no history of autoimmune disorders—the real-world application is yet to be defined. Nevertheless, in 2016, the landscape of NSCLC has irrevocably changed. The treatment paradigm will now include PD-L1 testing for every patient with advanced NSCLC at diagnosis. For patients with PD-L1 expression ≥ 50%, first-line pembrolizumab has displaced platinum-based doublet chemotherapy and represents the new standard of care. ✦
References
1. Brahmer JR, Drake CG, Wollner I, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol. 2010;28:3167-3175.
2. Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non- Small-Cell Lung Cancer. N Engl J Med. 2015;373:1627- 1639.
3. Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med. 2015;373:123-135.
4. Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387:1540- 1550.
5. Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372:2018-2028.
6. Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab versus Chemotherapy for PD-L1- Positive Non-Small-Cell Lung Cancer. N Engl J Med. 2016 Oct 8. [Epub ahead of print]