By Edgardo S. Santos Castillero, MD, and Roberto I. Lopez, MD
In August 2016, the 7th Latin America Conference on Lung Cancer (LALCA) was held in Panama City, Republic of Panama. More than 500 healthcare professionals gathered to discuss the latest advances in prevention, detection, diagnosis, and treatment for lung cancer. This congress, which was sponsored by the IASLC, takes place every 2 years in different countries of the region. The meeting was led by Drs. Roberto I. Lopez, Local Chair, and Edgardo S. Santos Castillero, International Chair. This was the first LALCA meeting where trainees from different disciplines (thoracic surgery, radiation oncology, pathology, radiology, pulmonary, and medical oncology) had the chance to attend the “School of Thoracic Oncology”; this 1-day workshop led by Drs. Luis E. Raez and Suresh Ramalingam offered trainees the opportunity to interact and learn the basics of lung cancer diagnosis, prognosis, and pathology, as well as the role of surgery, radiation, and new targeted therapies, at no cost. In addition, LALCA Panama opened its doors to cancer patients and their relatives. This new Patient Advocacy session provided experts in the field with the opportunity to talk to patients and families about current treatments available, the importance of genetic testing, drug availability, costs, and other topics. Two lung cancer survivors with stage IV disease also participated as guest speakers and shared their experiences fighting lung cancer. Both sessions were well received by the participants. The LALCA meeting agenda also included educational conferences, a roundtable session to discuss major topics with experts in the field, poster display and discussion, and oral presentations.
Several significant abstracts were presented at the conference. In an international effort involving several Latin America countries, Dr. Oscar Arrieta (Mexico) et al presented their results on acquired resistance, clinical characteristics, and survival outcomes of patients whose disease progressed on epidermal growth factor receptor (EGFR)-tyrosine kinase inhibitors (TKIs) in EGFR mutant (+) lung adenocarcinoma among Hispanics. In this prospective registry study, 34 patients with acquired resistant to TKI were identified over a period of 4 years. After progression, all patients were re-biopsied; similar to what has been found in Caucasian patients, T790M was the most common cause of acquired resistance (47.1%), followed by PI3K mutations (14.7%), EGFR amplification (14.7%), MET amplification (8.8%), and others. Interestingly, these patients were still sensitive to other therapies after progression (overall response rate [ORR], 47.1%).
Today, liquid biopsy represents an alternative besides tumor tissue specimens to discover druggable molecular pathways. Dr. David Gandara (US) et al presented the results of largest cohort of plasma next generation sequencing (NGS) performed in advanced non-small cell lung cancer (NSCLC) and its clinical correlations. Guardant360, a highly accurate, deep-coverage circulating tumor DNA (ctDNA) plasma NGS test, was used to genotype 5206 advanced-stage NSCLC patients. In this cohort, 85% of samples were ordered at clinical progression and 15% at baseline in patients who had insufficient tumor specimen for analysis. Somatic ctDNA alterations were detected in 86% of cases; most common ones included EGFR (25%) and KRAS (17%).
Today, liquid biopsy represents an alternative besides tumor tissue specimens to discover druggable molecular pathways
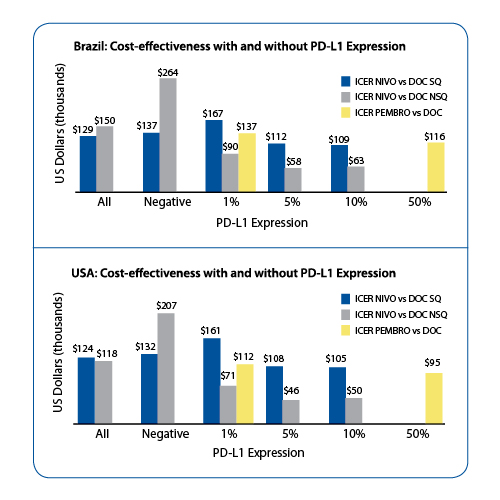
Among driver oncogenes, the alteration pattern was highly consistent with those previously described in the tissue sequencing compendia (e.g., TCGA). Dr. Gandara pointed out that the accuracy of ctDNA sequencing (PPV) found in the 229 consecutive samples matched with their tissue testing report was 100% for EGFR L858R, 98% for EGFR exon 19 deletion, 96% for ALK, RET or ROS-1 fusions, and 100% for KRAS mutations. In 63% (229/362) of consecutive NSCLC cases, tissue was insufficient or undergenotyped. More importantly, alterations not previously identified were found via ctDNA NGS in 24% (51/229) of these cases. For those patients who were tested at progression, resistance mutations in 1111 EGFR mutant NSCLC cases were identified via ctDNA NGS at frequencies consistent with published literature: 47% had EGFR T790M, 5% MET amplification, 5% ERBB2 amplification, 3% NF1 inactivation and RB1 inactivation, 2.5% PTEN inactivation, 1.9% KRAS mutations, 1.8% BRAF mutations, and others. Dr. Gandara concluded that ctDNA NGS is capable of identifying new druggable alterations at progression and when tumor tissue is insufficient. This technology also opens the possibility of serial testing for evolution of resistant alterations in lung cancer. Among the highest rank abstracts, we report a study on cost-effectiveness of immune checkpoint inhibitors in NSCLC relative to PD-L1 expression. This analysis was presented by Dr. Gilberto Lopes (Brazil) et al. With the advent of checkpoint blockade, there is tremendous pressure from health insurance companies, government, patient advocacy groups, and regulatory authorities due to the high cost of these novel agents and the impact on the healthcare system. Conversely, these agents have also been shown to improve overall survival in firstline (e.g., KEYNOTE 024 trial) and second- line therapy (e.g., CheckMate 017, CheckMate 057, KEYNOTE 010 trials) in advanced NSCLC patients. A decisionanalytic model to determine the costeffectiveness of PD-L1 assessment and second-line therapy with nivolumab or pembrolizumab vs docetaxel was developed by the investigators. The model used outcomes data from 3 randomized clinical trials (RCTs) and drug acquisition costs from the US. For squamous NSCLC, the incremental quality-adjusted life-years (QALY) of nivolumab was 0.23. The incremental cost-effectiveness ratios (ICER) was USD $128,000. PD-L1 expression improved incremental QALY only for patients with PD-L1 > 5% and > 10%. Among all non-squamous NSCLC, the incremental QALY of nivolumab was 0.12 and ICER was USD $121,000. PD-L1 expression improved incremental QAYL for patients with PD-L1 > 1%, > 5%, > 10%. This led to a drop in the ICER in all 3 groups of patients stratified by PD-L1 expression. KEYNOTE-010 study included patients with at least 1% or higher PD-L1 expression; the incremental QALY was 0.13 for pembrolizumab. The ICER was USD $116,000. For those patients whose tumors had a PD-L1 > 50%, the incremental QALY was improved by 18% and the ICER dropped to USD $98,000 (Figure 1). Lopes concluded that the use of PD-L1 expression as a biomarker for patient selection improves the cost-effectiveness of treatment with PD-1 inhibitors and has the potential to decrease the total cost of therapy with these agents in both Brazil and in the United States; however, this improvement comes at a cost. By using PD-L1 as a biomarker, fewer patients are treated and the total life years gained is likely to be lower than if we treat all patients. ✦











