The College of American Pathologists (CAP), International Association for the Study of Lung Cancer (IASLC), and Association for Molecular Pathology (AMP) published online in April 2013 the original “Molecular Testing Guideline for Selection of Lung Cancer Patients for EGFR and ALK Tyrosine Kinase Inhibitors” in Archives of Pathology & Laboratory Medicine, Journal of Thoracic Oncology, and The Journal of Molecular Diagnostics, respectively. This guideline contained 15 evidence (grade A/B) based recommendations for molecular analysis of lung cancers. The major recommendations were (1) to use molecular testing for epidermal growth factor receptor (EGFR) mutations and anaplastic lymphoma kinase (ALK) fusions to guide patient selection for therapy with an EGFR or ALK inhibitor, respectively, in all patients with advanced-stage adenocarcinoma, regardless of sex, race, smoking history, or other clinical risk factors, (2) to prioritize EGFR and ALK testing over other molecular predictive tests, and (3) to address how the testing should be performed.
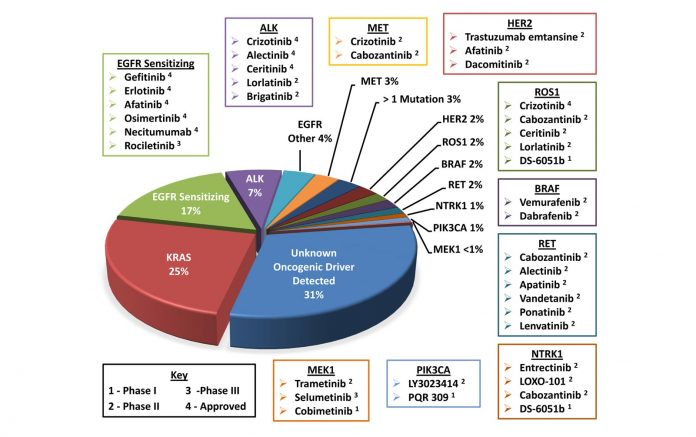
Scientific knowledge on new biomarkers for targeted therapy like ROS1, RET, BRAF, and MET and new technology like next-generation sequencing continues to advance at an incredible pace due to discoveries from basic science, translational science, and clinical trials. Consequently, the Molecular Testing Guideline needed to be updated to communicate emerging clinical standards and to leverage the latest breakthroughs in lung cancer research. Moreover, a guideline that has not been reaffirmed and updated after 5 years or more is no longer considered current. The guideline revisions are designed to provide state-of-the-art molecular testing recommendations for pathologists, oncologists, and other cancer and molecular diagnostic laboratory professionals involved in the management of lung cancer.
The co-chairs Philip T. Cagle, MD, on behalf of CAP, Yasushi Yatabe, MD, PhD, on behalf of IASLC, and Neal Lindeman, MD, on behalf of AMP, along with a multidisciplinary expert panel from all three organizations, developed the following key questions about update and revision at a 2-day face-to-face meeting:
• What other genes, previously not addressed, should be tested in lung adenocarcinoma?
• Is immunohistochemistry reliable for screening for ALK translocations?
• What are the types and rates of secondary resistance in patients who are undergoing treatment with targeted tyrosine kinase inhibitors?
• What are the clinical performance characteristics of circulating DNA/ CTC in plasma when used for diagnosis of primary lung adenocarcinoma or relapse?
• Are there biomarkers that are predictive of clinical outcome in squamous and small cell lung carcinomas?
• What key considerations are there regarding next-generation sequencing panels targeting key genes or regions of interest?
To address these questions, an extensive literature search strategy, containing inclusion and exclusion criteria, was developed, and an unbiased review of published experimental literature since 2013 was executed. Over 1,650 abstracts were captured in the initial literature search, and more than 450 of these were moved on to a full-text review. Tables were developed from extracted data for approximately half of the studies that went through the full-text review. Expert panelists then evaluated the extracted data to develop draft recommendations that addressed the key questions. The draft recommendations were then posted online for 5 weeks following multiple announcements from all three organizations to garner public comments, which were fully evaluated and discussed by the expert panel and co-chairs. The recommendations are now in the process of being revised based on the feedback from the open comment period. The final recommendations, once the manuscript is completed, will be jointly approved and once again published in Archives of Pathology & Laboratory Medicine, Journal of Thoracic Oncology, and The Journal of Molecular Diagnostics, with an expected publishing date in late 2016 or early 2017.
Molecular testing for the selection of optimal therapy for patients with lung cancer remains as crucial as today as when the “Molecular Testing Guideline for Selection of Lung Cancer Patients for EGFR and ALK Tyrosine Kinase Inhibitors” was originally published. However, what has shifted is the clinical situation as a result of the tremendous effort of researchers and patients all over the world. Therefore, the expanded list of genes and new methods in the updated Molecular Testing Guideline are both clinically relevant and necessary. —Yasushi Yatabe, MD, PhD Expert Panel Co-Chair, Lung Guideline Update Project