Next-generation sequencing (NGS) technology has revolutionized genomic and genetic research as it fulfills its potential to tailor the treatment of cancer patients in general and non-small cell lung cancer (NSCLC) patients in particular to their cancer genome alterations. The role of NGS in drug development and clinical trials varies worldwide in response to regional and national support and organization. IASLC Lung Cancer News invited experts from France, Germany, and China to summarize their NGS molecular diagnostics networks, and to comment on the implications and accomplishments of their programs for the development of treatments for patients with thoracic malignancies.
NGS Lung Cancer Genotyping Program in France
By Frédérique Nowak, MS, PhD, and Jean-Charles Soria, MD, PhD
The development of precision medicine in oncology requires that molecular diagnostics be performed on as broad a scale as possible so as to ensure health care equity. To this end, as early as 2006, the French National Cancer Institute (INCa) and the French Ministry of Health decided to set up a specifıc molecular testing organization and created a nationwide network of 28 molecular genetics centers located throughout France.1 The centers were selected through competitive calls for proposals managed by INCa in 2006 and 2007. Each molecular genetics center is composed of several university hospital and cancer center laboratories with complementary expertise in all the DNA-based and RNA-based techniques required for molecular testing.
These centers perform molecular tests for all patients in their region, irrespective of the institutions (university hospitals, cancer centers, local public hospitals or private institutions) where the patients are being treated. The corresponding costs are covered by INCa and the French Ministry of Health.
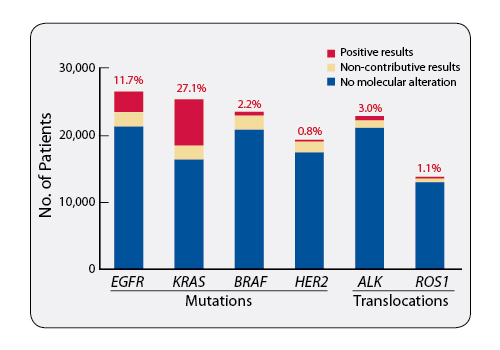
The dynamics of access to molecular testing in France is monitored by INCa. In 2015, more than 60,000 cancer patients underwent molecular predictive tests. Among the 26,600 patients with advanced-stage or metastatic nonsquamous NSCLC who underwent EGFR screening in 2015, 3,100 (11.7%) had tumors with an EGFR mutation and were therefore eligible for EGFR-TKI treatment (Figure 1). Moreover, the 28 molecular genetics centers in 2015 identified 700 patients with an ALK rearrangement, 520 patients with a BRAF mutation, and 160 patients with a ROS1 rearrangement, giving them the possibility of access to, respectively, ALK inhibitors, BRAF inhibitors, and crizotinib, which is available in France for ROS1-positive patients.
Beyond offering widespread access and nationwide coverage, achieving, securing, and maintaining quality are crucial. For this purpose, INCa fosters multidisciplinarity and the development of a collaborative network between centers. INCa has also set up a quality assurance program based on the publication of guidelines and on the organization of national External Quality Assessment rounds for the tests that have a major therapeutic impact on patients.
The increasing number of actionable molecular abnormalities that have to be screened for each patient has led the centers to turn towards technologies allowing multiplex analysis in an “allin- one” approach. Since 2013, INCa has supported the implementation of targeted Next Generation Sequencing (NGS) as part of routine clinical practice. The targeted analysis of a panel of genes is an adequate short-term approach that can be implemented within the existing organizational framework. Nevertheless, laboratories need to adapt to constantly evolving technologies, which is extremely challenging. Moreover, they need to acquire specific expertise in bioinformatics for data analysis. To this end, working groups have been set up by INCa to foster interinstitutional communication and to facilitate the drafting of guidelines. Reference teams in bioinformatics have also been funded to support wet labs and their “embedded” bioinformaticians through network animation, release of validated data analysis tools, and training. The objective is to shift progressively from standard approaches towards targeted NGS for all patients by the end of 2016.
The next challenge is to make the most of the additional information obtained by targeted NGS in routine practice. New tools are required to assess the clinical significance of new variants. In this context, collecting information about rare mutations in relation to patients’ followup data becomes critical. Molecular Tumor Boards are also being organized more widely in the French territory to help clinicians in interpreting molecular data. Finally, targeted NGS allows an opportunity to offer access to experimental compounds; its broad use will be linked to a strategy aimed at improving accrual onto multicentric genomics- driven clinical trials. Daily testing for molecular subsets in metastatic NSCLC allows for, at the national level, creation of readily available cohorts that can then be referred to recruiting centers. In that regard, France was the top recruiter of the dabrafenib/trametinib phase II trial in BRAF V600 (+) NSCLC. In parallel, INCa is supporting the ACsé program, which offers, within the secure framework of a clinical trial, broad access to targeted therapies outside their approved marketing indication.2 Nevertheless, there is clearly room to grow. Data from the Biomarker France study show we can increase the enrollment of patients carrying “actionable” molecular alterations onto studies evaluating experimental compounds.3
Such an organization for the nationwide provision of molecular tests is facing a fast-evolving scientific, medical, and technological environment. In this context, implementation of targeted NGS is only one step. The recent development of checkpoint inhibitors also holds the potential for improving patient outcomes. Nevertheless, specific predictive biomarkers are required for the identification of patients most likely to respond to treatment, and they are currently under development. As these assays enter into clinical practice, it will require the accurate integration of new expertise within the existing organizational framework to provide a complete service offering for precision medicine.
References
1. Nowak F, Soria JC, Calvo F. Tumour molecular profiling for deciding therapy-the French initiative. Nat Rev Clin Oncol. 2012; 9:479-486.
2. Buzyn A, Blay JY, Hoog-Labouret N, et al. Equal access to innovative therapies and precision cancer care. Nat Rev Clin Oncol. 2016; 13: 385-393.
3. Barlesi F, Mazieres J, Merlio JP et al. Routine molecular profiling of patients with advanced non-small-cell lung cancer: results of a 1-year nationwide programme of the French Cooperative Thoracic Intergroup (IFCT). Lancet. 2016; 387:1415-1426.
Network of Genomic Medicine Lung Cancer: A German Perspective
By Cynthia L. Kryder, MS, as described by Anne Schultheis, MD
Our growing understanding of the molecular basis of cancer has led to an evolution in personalized medicine and has created the need to offer molecular diagnostics to all patients with advanced lung cancer. To ensure patients with lung cancer in Germany have access to individualized therapies based on a comprehensive molecular analysis of their tumors, the Cologne Lung Cancer Group (LCGC) at the Center for Integrated Oncology (CIO) at the University Hospital Cologne established the Network Genomic Medicine (NGM) Lung Cancer in March 2010. The NGM Lung Cancer represents an interdisciplinary collaboration between lung cancer physicians and research scientists at the University Hospital Cologne and local hospitals, pathologists, and private oncology practices throughout Germany to deliver precision treatment to patients nationwide. The NGM is unique in that the German government has had no role in its creation and has no involvement with regard to patient access, implementation, or oversight.
“The network aims to provide a comprehensive genomic workup for any patient with a nonresectable, malignant lung tumor and to direct them to the appropriate treatment or clinical trial regardless of their geographic location or reimbursement status,” explains Anne Schultheis, MD, Institute for Pathology, University of Cologne. Dr. Schultheis notes that, since 2014, a portion of the costs for the genomic analysis has been reimbursed by health insurers. Any unreimbursed cost is borne by the NGM.
Access to molecular testing through the NGM is limited only by awareness among local oncologists and pathologists. “Patients’ tumor biopsies generally are sent first to local pathologists who, if they, or the treating oncologists, are aware of the NGM, will pass on the biopsies of patients with nonresectable, advanced lung tumors so that we can perform a comprehensive genomic analysis,” says Dr. Schultheis. Once in the network, patients with lung cancer gain access to the latest treatments and clinical trials.
The NGM is unique in that the German government has had no role in its creation and has no involvement with regard to patient access, implementation, or oversight.
Molecular analysis of tumor tissue is centralized at either the main laboratory at the Institute for Pathology at the CIO, University Hospital Cologne, or at a second regional laboratory. Directed by Reinhard Büttner, MD, the pathology team focuses on molecular diagnosis as well as on the determination of predictive markers, with special interest also in immunotherapy. When actionable mutations are detected and approved therapies are available, subsequent treatment is decentralized, enabling patients to receive treatment close to home. For patients with detected mutations for which no drug has yet been approved, NGM attempts to direct them to an appropriate clinical trial at a centralized location.
With its roots in academia, NGM can take advantage of the latest research findings from the experts at the LCGC study center. Led by Jürgen Wolf, MD, the LCGC study program develops clinical trials to treat genetically defined subgroups of lung cancer and to optimize immunotherapy in coordination with work groups in translational cancer genomics, molecular pathology, and molecular imaging at the University of Cologne. This ongoing exchange of information allows NGM to offer clinical studies to most patients with lung cancer who have a therapeutically relevant genetic aberration in their tumor.
Molecular testing is performed under very strict laboratory procedures. To detect all relevant changes in lung cancer tumor cells, the NGM has designed its own molecular diagnostic platform covering all known driver mutations. The current lung cancer-oriented platform is updated regularly based on new research findings and includes, among others, mutations in the genes EGFR, KRAS, BRAF, PIK3CA, HER2, p53, MET, and DDR2; translocations in ALK, RET, and ROS1; and amplifications in HER2, MET, and FGFR1. The NGM uses a multiplex test in combination with deep sequencing to detect rare gene mutations in the smallest tissue samples. This approach involves multiple techniques such as next-generation sequencing, immunohistochemical detection of protein expression, and fluorescence in situ hybridization (FISH).
In the future, the NGM plans to convert to a hybrid-capture genetic analysis technique that will increase the number of mutations that can be detected. This approach involves an analysis of multiple oncogenes and multiple genetic aberrations, not only for lung cancer but also for other malignant tumors. This process can potentially lead to the identification of new molecular markers that may be clinically relevant.
Over the past 6 years, NGM has evolved from providing services to patients in only one geographic region, North Rhine-Westphalia, to offering comprehensive genomic workups to any patient in the country. Currently, NGM is Europe’s largest platform for molecular diagnostics. “Each year NGM screens about 4,500 patients with lung cancer (and about 2,000 patients with other tumors, such as melanoma, ovarian cancer, or gastrointestinal tumors) for genomic aberrations,” says Dr. Schultheis. “This number represents 35% of all newly diagnosed patients with lung cancer in Germany.” With such widespread access, increasing awareness among patients and providers becomes a challenge. “More hospitals and private oncologists need to be aware of the network and the importance of precision therapies,” Dr. Schultheis acknowledges.
The close cooperation between the NGM, the LCGC, and the University of Cologne as well as the partnerships with healthcare providers throughout the country has been a key to NGM’s success. “As an interdisciplinary network, we are working hand in hand to accomplish our goal of delivering personalized medicine to every patient in Germany with advanced lung cancer,” says Dr. Schultheis.
Current State of NGS-Based Lung Cancer Genotyping in China
By Yang Shao, PhD
The clinical application of next generation sequencing (NGS) first kicked off in the field of prenatal genetic screening for birth defects in China around 2012. Four years later, it has become part of a routine program adopted by hospitals and expecting parents, and it has transformed into a billion-dollar industry. NGS-based genetic tests first appeared in the oncology clinic in 2014, with the lung cancer field one of the earliest adopters, for several reasons. First, the wide use of targeted therapies such as EGFR, ALK, and ROS1 inhibitors poses real needs for multi-gene-based genotyping tools.1,2,3 Second, molecular interactions between the tumors and the drugs such as sensitizing and resistance mechanisms are relatively better understood in lung cancer; identifying markers of sensitivity and resistance therefore provides a scientific basis for clinical decision-making. Third, lung cancer is the most prevalent cancer type in China and attracts a lot of industry attention. Comparing to Sanger sequencing, qPCR, or FISH-based techniques, NGS-based genetic testing offers several advantages, including higher sensitivity/ specificity, the capacity to provide a more well-rounded view of the tumor genetics, and the ability to offer a one-test-for-alldrugs approach.4 NGS also allows us to best utilize limited tissue samples, which is a frequent challenge. In addition, delineating a group of genetic alterations such as MET exon 14 skipping5 and rare EGFR sensitizing mutations6 can only be accomplished with NGS-based tests.
The majority of the commercially available NGS-based tests are gene panels ranging from several genes to several hundred genes; the price ranges from 7000RMB to 18,000RMB (approximately $1,050 USD to $2,800 USD). Small gene panels usually focus on core NCCN recommendation genes, while large panels can encompass all known cancer-related genes. For example, “Sangtinel,” a popular liquid biopsy test offered by Geneseeq Technology Inc., China, includes 416 genes and several gene fusion events. Several controversies exist in the commercial implementation of such tests: 1) How to best control and monitor the quality of various tests commercially offered? 2) Should the test exist as a laboratory- developed test (LDT) or as part of a commercial diagnostic kit? and 3) How large should the gene panels be? These questions have been intensively debated by experts in the field over the past year.
NGS combined with liquid biopsy is another nascent and rapidly emerging field in China. Liquid biopsy tests exploit the presence of circulating tumor DNA in the blood. Since it is minimally invasive, liquid biopsy allows multiple, repeat sampling from the same patient, and it opens up the chance to gauge genetic evolution, to monitor for residual disease, to determine treatment efficacy, and to identify the development of therapeutic resistance. Oncologists in China have generally accepted the idea of liquid biopsy testing and have employed this technology on some endstage patients from whom tissue biopsies are not feasible. The recent approval of cobas EGFR Mutation Test v2 (Roche) by the FDA is likely to attract more interest and accelerate development in this area.
Several obstacles challenge the nationwide adoption of NGS-based tests in China. Cost is still a major issue. Most genetic tests are not covered by governmental health insurance in China, and most patients end up paying out-of-pocket. Understanding and making use of the vast amount information generated by NGS-based tests is another. Many oncologists feel overwhelmed by the reports—systemic elucidation of clinically relevant information is important. Regulatory approval and quality control are also key in guiding the field. With Precision Medicine being part of the central 13th 5-Year Plan commencing this year, the Chinese government is taking a positive view toward developments in this area, even though, so far, none of the NGS-based tests have been officially approved by the China Food and Drug Administration, and proper and minimal guidelines will be required to ensure the quality of various available commercial products.
Last, pharmaceutical companies have been late adopters of this technology. Most clinic trials do not routinely require or accept NGSbased tests as entry criteria, though the potential benefits from this comprehensive technique are clear.
In summary, NGS-based genotyping is a rapidly emerging and promising field in China that can bring tangible clinical benefits to patients and fulfill the vision of Precision Medicine, particularly in thoracic oncology. It has the potential to turbocharge translational research and speed up therapeutic innovation.
References
1. Sharma SV, Bell DW, Settleman J, Haber DA. Epidermal growth factor receptor mutations in lung cancer. Nat Rev Cancer. 2007; 7:169-181.
2. Sullivan I, Planchard D. ALK inhibitors in non-small cell lung cancer: the latest evidence and developments. Ther Adv Med Oncol. 2016; 8:32-47.
3. Shaw AT, Solomon BJ. Crizotinib in ROS1-rearranged non-small-cell lung cancer. N Engl J Med. 2015; 372: 683-684.
4. Frampton GM, Fichtenholtz A, Otto GA, et al. Development and validation of a clinical cancer genomic profiling test based on massively parallel DNA sequencing. Nat Biotechnol. 2013; 31:1023-1031.
5. Zheng D, Wang R, Ye T, et al. MET exon 14 skipping defines a unique molecular class of non-small cell lung cancer. Oncotarget. 2016. [epub ahead of print] doi: 10.18632/oncotarget.9541
6. Stewart EL, Tan SZ, Liu G, Tsao MS. Known and putative mechanisms of resistance to EGFR targeted therapies in NSCLC patients with EGFR mutations-a review. Transl Lung Cancer Res. 2015; 4:67-81.