By Beth Eaby-Sandy, MSN, CRNP, OCN, Massey Nematollahi, RN, MScN, OCN, CON, Diana Borthwick, MSc, PGD, and Beth Ivimey, RN
United States of America
There are now 2 distinct immunotherapy drugs approved, nivolumab and pembrolizumab, for the treatment of NSCLC after failure of platinum-based chemotherapy. Both nivolumab and pembrolizumab produce similar response rates and toxicity profiles. However, the toxicity profiles differ substantially from what oncology nurses are accustomed to seeing.
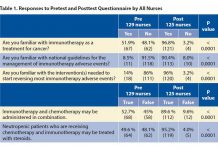
While fatigue, rash, and diarrhea may be mild common adverse events with these drugs, and are side effects that most oncology nurses or advanced practitioners are accustomed to troubleshooting, immune mediated toxicities such as colitis, pneumonitis, dermatitis, and endocrinopathies can be severe and, at times, life-threatening. Nurses now must learn the signs and symptoms of these new toxicities, as well as the very different management strategies required to control them.
Several nursing educational platforms such as Medscape, Clinical Care Options, OncLive, Meniscus, the Oncology Nursing Society (ONS), and the Advanced Practitioner Society of Hematology and Oncology (APSHO), as well as others, have launched continuing education programs aimed to teach nurses about these new therapies and their toxicities.
Canada
Currently, there are two immunotherapy drugs used in Canada: nivolumab and pembrolizumab, which both carry the same indications as in the United States in the second-line setting for metastatic NSCLC.
The key to successful treatment using this new class of agents is communicating with patients and providing ample education based on particular symptoms that the patient might develop.
Cancer Care Ontario (CCO), the British Columbia Cancer Agency (BCCA), and other cancer nursing organizations in Canada have created guidelines for managing these unique side effects as well as patient education information in lay language.
Most of the cancer centers have also established a callback program to patients on these therapies. This is an ideal way to capture all patients starting on immunotherapy and to monitor them throughout their treatment. These individuals are flagged in a computerized system and nurses call them at certain time points to perform an assessment over the phone.
Key questions are added in a dropdown list based on the organ and body system to be assessed. This is an efficient way to capture immune-mediate adverse events through electronic documentation at the right time and to provide necessary and timely interventions.
United Kingdom
Currently, there are no immunotherapy drugs approved in the UK, although both nivolumab and pembrolizumab have been submitted for approval to both the National Institute for Health and Care Excellence (NICE) and the Scottish Medicine Consortium (SMC). These drugs are available in the private setting, in clinical trials, and on compassionate use programs while the UK awaits decisions regarding approval from NICE and SMC.
Nurses are just beginning to gain experience in managing patients on these new drugs. However, initial impressions are positive. Nurses have reported improvements in patient quality of life, with minimal toxicities from these drugs. However, we must not be complacent, as these drugs can result in severe immune toxicities that can present at any point in the course of treatment. We have concentrated heavily on the nursing management of patients receiving immunotherapies at the National Lung Cancer Forum for Nurses (NLCFN) Conference held last year, and we are currently putting together an educational tool for nurses on immunotherapies.
Australia/New Zealand
In Australia and New Zealand, there is an expanded access program to use nivolumab for patients who have already received first-line chemotherapy and require second-line treatment. Pembrolizumab is available only to patients participating in clinical trials in Australia.
As far as educational resources for nurses and patients, we have relied heavily upon our melanoma nurse colleagues to educate and support us for the challenges coming our way as we start implementing this new class of drugs in the management of our metastatic lung cancer patients. We are very appreciative of the support given to us by the melanoma community.
For formal education support, the Australian Cancer Nurses Society (CNSA) has supported us with education days, as well immunotherapy components during our annual conferences, and immunotherapy education will be incorporated into our Australian Lung Cancer Conference in Melbourne this August.