By Denise R. Aberle, MD
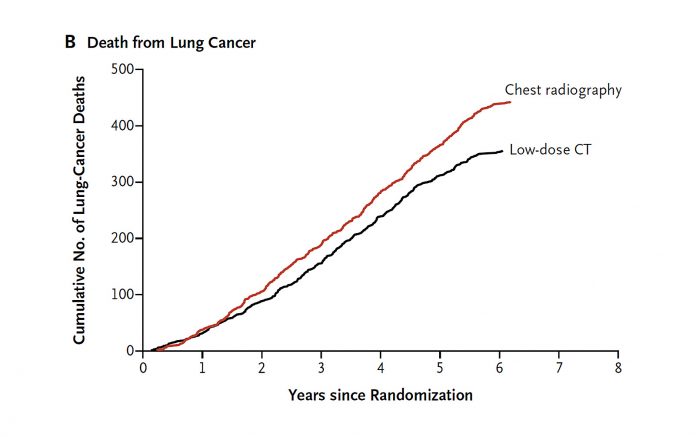
The seminal National Lung Screening Trial (NLST) showed that lung cancer screening using low dose computed tomography (LDCT) resulted in a 20% decrease in lung cancer mortality relative to chest radiography in older, heavy smokers. Additionally, there was a relative 6.7% decrease in all-cause mortality (due largely to improved lung cancer mortality), suggesting that LDCT screening did not significantly precipitate downstream mortality or that patients spared a lung cancer death did not die of comorbidities. These mortality reductions came at the cost of an overall 24% CT screen positivity rate, of which 96.4% were false positive results.1 Based on the NLST, CT screening for lung cancer is now a covered benefit in the United States by both third-party payers and Medicare, with the caveat that reimbursement is tied to submitting data on eligibility, screening, and outcomes to a central registry.2,3
The NLST enrolled individuals aged 55–74 years with a ≥30 pack-year current or former smoking history; for former smokers ≤15 years must have elapsed from the time they quit (YSQ). Both the United States Preventive Services Task Force (USPSTF), which determines policy for coverage by third-party payers, and the Centers for Medicare and Medicaid Services (CMS) currently base screening eligibility on the NLST, with each slightly extending the upper age limit of eligibility. However, the wisdom of constraining both pack-years of smoking and YSQ has been challenged,4,5 based upon data from SEER (Surveillance, Epidemiology and End Results) that indicate that only 26.7% of individuals with lung cancer would satisfy NLST eligibility criteria.6 Indeed, Pinsky found that current smokers with 20-29 pack-years had similar lung cancer risk to LDCT-eligible former smokers5; those with less smoking intensity were disproportionately women and racial/ethnic minorities. Several lines of evidence indicate that our current rulebased eligibility based on only age and smoking criteria are insufficient to identify the majority of individuals who get lung cancer. The National Comprehensive Cancer Network (NCCN) recommendations allow for younger smokers of lesser smoking intensity provided that they have at least one additional risk factor, such as family history of lung cancer, certain other cancers, underlying chronic obstructive pulmonary disease, or documented exposure to respiratory carcinogens7; limited studies in cohorts satisfying these criteria suggest that lung cancer rates are comparable to that of those enrolled on the NLST.8 Finally, models of lung cancer risk have been developed that improve on the NLST criteria; some expert groups suggest that risk prediction models are best suited to identify screening cohorts.9,10 By systematically extending eligibility criteria and capturing outcomes through national screening registries, we can ultimately improve lung cancer mortality and extend these benefits to a greater population who will be diagnosed with lung cancer.
The reduction of screening false positivity, which is among the greatest concerns with screening implementation, will require better definitions of screen positivity and strategies to map escalating management to degree of suspicion of the detected nodule. Following the NLST, in which small nodules 4–6 mm accounted for roughly half of positive screens, but less than 1% of lung cancers, the American College of Radiology established CT screening guidelines called LungRADS™.11 This guideline predicates management on nodule size, consistency, and changes over time, and has been shown in retrospective and limited prospective studies to increase the positive predictive value of a positive screen and reduce medical resource utilization at the likely expense of missed or delays in diagnosis of lung cancer.12,13 Mathematical models have been developed that may provide greater classification performance than rule-based approaches for indeterminate nodules, but our experience with diagnostic prediction models in the setting of the indeterminate nodule remains early.14
Additional unanswered questions with implementation are the optimal frequency and duration of screening, for which there are insufficient data. Annual screening was performed for 3 years in the NLST, and microsimulation models comparing annual, biennial, and triennial intervals have concluded that annual screening is most efficient. However, retrospective analyses suggest that in some low risk groups, such as those with negative baseline screens or repeat normal screens, annual screening may not be necessary.15 Nor are there any data regarding screening beyond 3 years. Answers to these questions may be attainable with long-term follow-up of cohorts of varying risk.
The NLST has changed public policy on lung cancer screening and provides for the early detection of lung cancer to reduce lung cancer mortality. Initial implementation in the US is largely patterned after the original NLST eligibility criteria. The current mandate for data submission to a national registry provides us with a critical opportunity to initially extend eligibility criteria to better define the risk profiles of the optimal screening cohort. The collection of outcomes data should also yield insights into diagnostic management in the setting of screen-detected nodules as well as the frequency and duration of screening. At a minimum, we have a road map to refine screening practice and incorporate new ideas as they emerge. ✦
References
1. National Lung Screening Trial Research Team, Aberle DR, Adams AM, Berg CD, et al. Reduced lung-cancer mortality with low-dose computed tomography screening. N Engl J Med. 2011;365:395-409.
2. Moyer VA; US Preventive Services Task Force. Ann Intern Med. 2014; 160:330-338.
3. Medicare coverage. https://www.medicare.gov/coverage/lung-cancer-screening.html. Accessed April 25, 2016.
4. Pinsky PF, Zhu CS, Kramer BS. Lung cancer risk by years since quitting in the 30+ pack year smokers. J Med Screen. 2015;22:151-157.
5. Pinsky, Kramer BS. Lung cancer risk and demographic characteristics of current 20-29 pack-year smokers: Implication for screening. J Natl Cancer Inst. 2015;107(11). pii:djv226. doi:10.1093/jnci/ djv226.
6. Pinsky PF, Berg CD. Applying the National Lung Screening Trial eligibility criteria to the US population: what percentage of the population and of incident lung cancers would be covered? J Med Screen. 2012;19:154-156.
7. NCCN Clinical Practice Guidelines in Oncology. Lung Cancer Screening Version 2.2016. https://www.nccn.org/professionals/physician_gls/f_guidelines.asp. Accessed April 25, 2016.
8. McKee BJ, Hashim JA, French RJ, et al. Experience with a CT screening program for individuals at high risk for developing lung cancer. J Am Coll Radiol. 2016;13(2 Suppl): R8-R13.
9. Tammemagi MC, Katki HA, Hocking WG, et al. Selection criteria for lung-cancer screening. N Engl J Med. 2013;368:728-736.
10. Tammemagi MC. Application of risk prediction models to lung cancer screening: a review. J Thorac Imaging. 2015;30:88-100.
11. Lung CT Screening Reporting and Data System (LungRADS™). http://www.acr.org/Quality-Safety/Resources/LungRADS. Accessed April 25, 2016.
12. Pinsky PF, Gierada DS, Black W, Munden R, Nath H, Aberle D, Kazarooni E. Performance of Lung-RADS in the National Lung Screening Trial: a retrospective assessment. Ann Intern Med. 2015; 162:485-491.
13. McKee BJ, Regis SM, McKee AB, Flacke S, Wald C. Performance of ACR Lung-RADS in a clinical CT lung screening program. J Am Coll Radiol. 2015;12:273-276.
14. McWilliams A, Tammemagi MC, Mayo JR, et al. Probability of cancer in pulmonary nodules detected on first CT screening. N Engl J Med. 2013;369:910-919.
15. Patz ER Jr, Greco E, Gatsonis C, Pinsky P, Kramer BS, Aberle DR. Lung cancer incidence and mortality in National Lung Screening Trial participants who underwent low-dose CT prevalence screening: a retrospective cohort analysis of a randomized, multicenter, diagnostic screening trial. Lancet Oncol. 2016. pii:S1470-2045(15)00621-X. doi:10.1016/S1470-2045(15)00621-X.