By Erik J. MacLaren, PhD
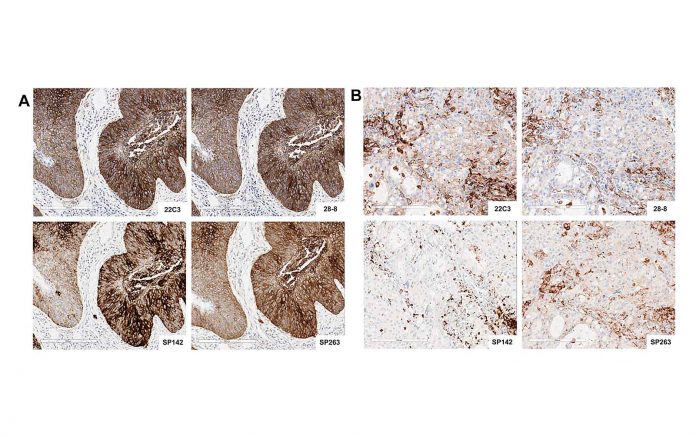
Immunotherapy with anti-PD-L1/PD-1 antibodies is rapidly changing the therapeutic landscape for patients with non-small cell lung cancer (NSCLC). However, selecting the most optimal patient population for those treatments remains a challenge. All pharmaceutical companies with a therapeutic antibody are pursuing a PD-L1 immunohistochemistry (IHC) assay either as a “companion” diagnostic or as a “complementary” diagnostic test. The challenge for the community is that each company pursues a unique assay for each drug, leading to several different PD-L1 IHC assays on the market for the same group of drugs. To better understand the different PD-L1 IHC assays and how they compare to each other, four different pharmaceutical companies (e.g., BMS, Merck, AstraZeneca, and Genentech/Roche), two diagnostic companies (e.g., DAKO/Agilent and Ventana/Roche) and two academic organizations: the International Association for the Study of Lung Cancer (IASLC) and the American Association for Cancer Research (AACR), established the “PD-L1 IHC Blueprint Project,” which aims to compare the four “distinct” PD-L1 IHC assays used in association with the drugs; Nivolumab (BMS), Pembroluzimab (Merck), Durvalumab (AstraZeneca), and Atezolizumab (Genenetech/Roche). The early results of this effort (feasibility component) were presented by Fred R. Hirsch, MD, PhD, University of Colorado and CEO of IASLC Hirsch FR et al: The PD-LI IHC Blueprint Project, AACR 2016).
Observations from the “PD-L1 IHC Blueprint Project” demonstrated analytical similarities and differences between the four assays; while three assays (e.g., clones 22C3, 28-8, and SP 263) were quite similar in PD-L1 expression, one assay (e.g., SP 142) was different. However, when clinical diagnostic paradigms were compared, the study demonstrated salient differences, which could lead to erroneous classification of PD-L1 status when related to the different agents under investigation. Thus, the consortium recommended that until more scientific data are gathered, each assay should be applied as recommended to its specific drug, and “mixing and matching” of assays might lead to erroneous classification of the patient tumor’s PD-L1 status.
A phase II of the PD-L1 IHC Blueprint Project is being planned as a validation study; this will include a much larger series of NSCLC tumors. ✦










