By Professor John K. Field MA, PhD, BDS, FRCPath
The advent of lung cancer computed tomography (CT) screening was initiated by the Early Lung Cancer Action Project (ELCAP) group in 19991 with their seminal publication in Lancet. This focused attention on the possibility of introducing CT screening as an early detection strategy, resulting in the funding of the National Lung Screening Trial (NLST) >53,000 persons trial in 2002, which by all accounts has been the largest randomized controlled lung cancer trial ever undertaken. The NLST trial demonstrated a 20% relative reduction in lung cancer mortality in the CT-screened arm compared to the chest X-ray arm.2,3 IASLC held a special CT screening workshop just days after this publication at World Conference on Lung Cancer (WCLC) 2011, from which the 75-member working group published their set of recommendations and posed specific outstanding questions.4 This is the good news; however, it has taken some time for CT screening to be implemented in the US, and only after recommendations from the US Preventive Services Task Force on CT screening and Medicare’s agreement to fund screening, within specific criteria.5 In Europe, a number of smaller trials have been undertaken in Italy, Denmark, Germany, and more recently in the UK; the largest of the European trials is the NELSON, which was undertaken in the Netherlands and Belgium (RCT CT vs no scan).6 We eagerly await the outcome of the NELSON trial in 2016/17, which will potentially herald the implementation of CT screening in Europe. There is also considerable experience in lung cancer CT screening trials in Japan, and more recently in China and Australia.
The question may be asked why has CT screening not been readily implemented outside the US? Clearly, the US has an insurance-based health care system, which is different from Europe and many other parts of the world. However, the focus is not only on a mortality reduction but also on cost-effectiveness. The NLST cost-effectiveness worked out to $81,000 (CI $52,000- $186,000) per quality adjusted life year (QALY), but most likely reflects the more costly health care system in the States.7 Recently, the UK Lung Cancer Screening (UKLS) modelled the baseline screening cost-effectiveness at £8,466 per QALY, ($13,071 per QALY gained (CI $8,556-$19,405).8 Thus, even allowing for the UKLS modelling being based on the baseline CT scans, it falls comfortably within the acceptable UK National Institute for Health and Care Excellence (NICE) guidelines (£20,000- £30,000/QALY).
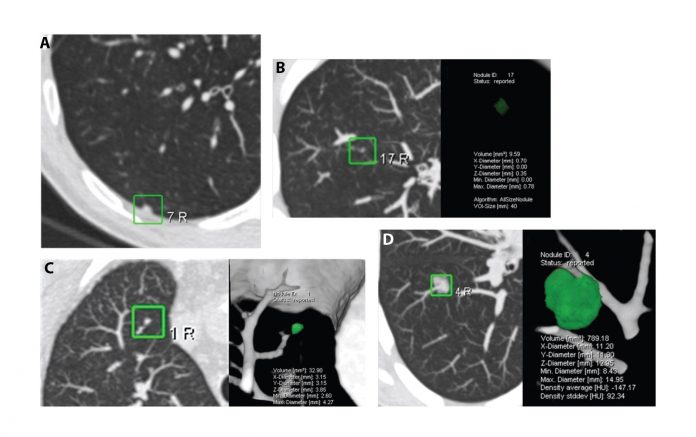
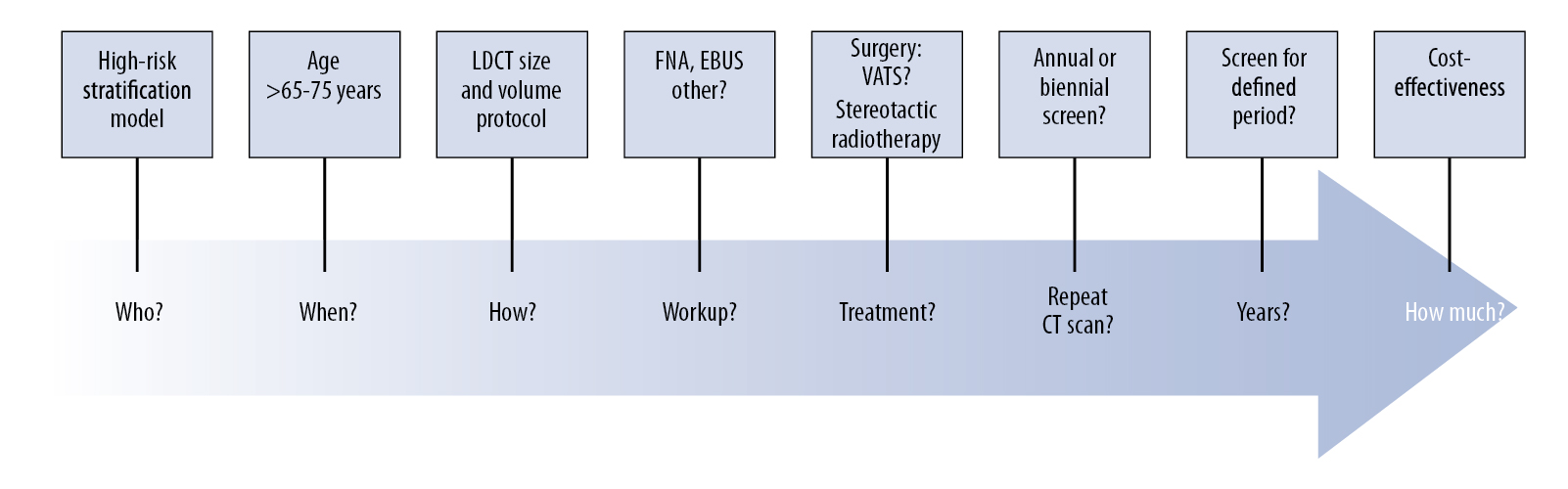
One of the outcomes of the IASLC CT screening workshop in 2011 was setting up the IASLC Strategic Screening Advisory Committee (SSAC), which has hosted 1-day workshops at WCLC 2013 in Australia and WCLC 2015 in Denver. These SSAC workshops have provided an opportunity to collegially involve global experts in discussing the outstanding questions concerning the practical issue of implementation, radiologic protocols, management of indeterminate nodules using volumetric analysis and volume doubling time (Figure 1), as well as considering the screening intervals and the patients’ lifetime involvement in future CT screening programs as outlined in a series of questions in Lancet in 2013 (Figure 2).9 We now need to focus on identifying the “hard-to-reach” communities and use the risk prediction models that have been demonstrated to successfully guide the selection of high-risk individuals for lung cancer CT screening programs. Looking to the future, the development and incorporation of molecular-genetic risk biomarkers into clinical-epidemiologic risk models will be a major research focus in the coming years. Currently, there is little discussion in the literature as to how the implementation of future CT screening programs will affect health care service delivery and how we will develop accreditation systems for the next generation of radiologists involved in CT screening programs. In this inaugural issue of IASLC Lung Cancer News, an overview of the current status of CT screening has been provided. Future issues of IASLC Lung Cancer News will focus on specific CT screening questions, which still need to be resolved, to continuously improve lung cancer early detection. The IASLC SSAC Workshops and meetings will take the lead on behalf of the Association to move this forward.
References
1. Henschke CI, McCauley DI, Yankelevitz DF, et al. Early Lung Cancer Action Project: Overall design and findings from baseline screening. Lancet. 1999; 354: 99-105.
2. Aberle DR, Berg CD, Black WC, et al. The National Lung Screening Trial: Overview and study design. Radiology. 2011; 258:243-253.
3. National Lung Screening Trial Research Team, Aberle DR, Adams AM, et al. Reduced lungcancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011; 365:395-409.
4. Field JK, Smith RA, Aberle DR, et al. International Association for the Study of Lung Cancer Computed Tomography Screening Workshop 2011 Report. J Thorac Oncol. 2012;7: 10-19.
5. de Koning HJ, Meza R, Plevritis SK, et al. Benefits and harms of computed tomography lung cancer screening strategies: A comparative modeling study for the U.S. Preventive Services Task Force. Ann Intern Med. 2014; 160: 311- 320.
6. van Klaveren RJ, Oudkerk M, Prokop M, et al. Management of lung nodules detected by volume CT scanning. N Engl J Med. 2009; 361: 2221-2229.
7. Black WC, Gareen IF, Soneji SS, et al, National Lung Screening Trial Research T. Cost-effectiveness of CT screening in the National Lung Screening Trial. N Engl J Med. 2014; 371:1793-1802.
8. Field JK, Duffy SW, Baldwin DR, et al. UK Lung Cancer RCT Pilot Screening Trial: Baseline findings from the screening arm provide evidence for the potential implementation of lung cancer screening. Thorax. 2016; 71: 161-170.
9. Field JK, Oudkerk M, Pedersen JH, Duffy SW. Prospects for population screening and diagnosis of lung cancer. Lancet. 2013; 382:732-741.










